Abstract
The mechanisms by which pulmonary granuloma formation is caused by administration of mycobacterial glycolipids such as trehalose dimycolate (TDM), lipoarabinomannan (LAM) and phosphatidylinositol mannosides (PIM) were investigated. When peritoneal and alveolar macrophages were stimulated with TDM, LAM and PIM in vitro, TDM exhibited the strongest tumour necrosis factor (TNF)-inducing activity. Responsiveness of macrophages from mice defected Toll-like receptor 4 (TLR4) was much higher than that of the wild-type mice. Although PIM and LAM also had a significant activity, LAM rather than PIM stimulated higher TNF-α production by alveolar macrophage. When mycobacterial glycolipids were injected as water-in-oil-in-water emulsion into mice via the tail vein, development of pulmonary granuloma in response to glycolipids were related closely to their TNF-inducing activity and TDM exhibited the strongest activity. Granuloma formation was observed not only in mice lacking interleukin (IL)-12 signalling but also interferon (IFN)-γ knock-out mice. Granuloma formation caused by glycolipids correlated with TNF-α levels in lungs. Administration of anti-TNF-α monoclonal antibody into TDM-injected IFN-γ knock-out mice decreased in granuloma formation, suggesting that development of pulmonary granuloma by mycobacterial glycolipids such as TDM is due to IFN-γ-independent and TNF-α-dependent pathway.
Keywords: granuloma formation, mycobacterial glycolipids, Mycobacterium tuberculosis
Introduction
Progressive granulomas are a characteristic hallmark of chronic mycobacterial infection as results of protective inflammatory responses initiated by Th1 cells and cytotoxic T lymphocytes (CTL). Lipid components present in the outer leaflet and the cell wall are implicated in granuloma pathogenesis. Trehalose 6, 6′-dimycolate (TDM), known as cord factor, is one of the characteristic lipids in the cell wall of Mycobacterium tuberculosis. TDM causes granuloma formation [1–3], inhibits tumour growth [4,5], increases antibody formation and induces angiogenesis [6] and thymic atrophy [7]. Furthermore, TDM shows increased sensitivity to endotoxin [8], activation of natural killer (NK) T cells and up-regulation of CD1d on macrophages [9]. Major biological activities are addressed by stimulation of macrophages by TDM followed by cytokine production such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-12 [2,10–13]. TDM also activates NK and Th1 cells to produce interferon (IFN)-γ. Among cytokines produced by TDM stimulation, TNF-α, IL-1β and IL-6 seem to relate closely to granuloma formation in the lung [14]. However, the mechanisms by which macrophages recognize TDM in innate immunity and present TDM as antigen to T cells still remains unclear.
Recent studies have addressed the pattern recognition of microbial components by Toll-like receptors (TLRs) expressing on phagocytes and other cells which play pivotal roles in the innate immunity. TLRs, which recognize their components of Gram-negative and Gram-positive bacteria, mediate cellular signalling to produce cytokines and chemokines [15–18]. Means et al. [19] showed that lipoarabinomannan (LAM) isolated from rapidly growing mycobacteria confer signalling to macrophages via TLR2 but not TLR4, although LAM isolated from M. tuberculosis and M. bovis bacillus–Calmette–Guerin (BCG) failed to cause cellular activation via TLR2. In contrast, Underhill et al. [20] showed that the cell wall fraction containing LAM from M. tuberculosis induced cellular responses via TLR2 expressing on phagocytes. The lipid part of LAM consists of phosphatidylinositol mannosides (PIM). The present study has focused therefore on the ability of these glycolipids isolated from M. tuberculosis on pulmonary granuloma formation in mice, when they were injected intravenously (i.v.) in a form of water-in-oil-in-water (w/o/w) emulsion.
Materials and methods
Glycolipids
TDM and PIM isolated from the M. tuberculosis H37Rv strain, as described previously [21,22], LAM from the M. tuberculosis Aoyama B strain purchased from Nacalai Tesque Co. (Kyoto, Japan) and lipopolysaccharide (LPS) from Salmonella abortus equi (a gift of Dr Chris Galanos, Max-Planck-Institute for Immunobiology, Freiburg, Germany) were used. The purity of TDM and PIM was confirmed by thin-layer chromatography (TLC) to be a single spot. LAM was analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Contamination by each other was not observed. W/o/w emulsions of test samples were prepared as described previously [8]. For in vitro TNF-α release from macrophages, TDM, PIM and LAM were dissolved in hexane, ethanol and 50 mM sodium carbonate buffer (pH 9·6), respectively. For coating each glycolipid, 50-µl aliquots were distributed into 96-well plates at a dose of either 1 or 10 µg/well and evaporated. In a case only of LAM, after evaporation plates were washed three times with 200 µl Dulbecco's phosphate-buffered saline (PBS)(–) to remove the Na salt.
Mice
Female C57BL/10ScSn (the wild-type, B10Sn), C57BL/10ScCr (TLR4-deletion and IL-12Rβ2-mutation at the STAT4 binding site, B10Cr), BALB/c, BALB/lpsd (C3H/HeJ-type TLR4-mutation) and IFN-γ knock-out (IFN-γ –/–, BALB/c background) mice were housed under specific pathogen-free conditions and injected intravenously (i.v.) with w/o/w emulsions of glycolipids at a dose of 10 µg per mouse to induce granuloma formation. All experiments were approved by the Animal Use Committee of Kitasato University School of Science.
Preparation of macrophages
Peritoneal macrophages from B10Sn and B10Cr mice, injected intraperitoneally (i.p.) with 2 ml of 10% proteose peptone broth 4 days previously, were obtained by washing the peritoneal cavity with 4 ml Hanks's balanced salt solution (HBSS). Cells were washed twice with PBS(–) and suspended into a complete RPMI-1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), 50 µM 2-mercaptoethanol (2-ME), 100 U/ml penicillin and 100 µg/ml streptomycin at a cell number of 5 × 106 cells/ml.
Alveolar macrophages from B10Sn mice were obtained by bronchoalveolar lavage with 10 ml PBS(–) containing 0·2% ethylenediamine tetraacetic acid (EDTA). Cells were washed twice with PBS(–) and suspended into the complete RPMI-1640 medium at a cell number of 5 × 106 cells/ml.
Release of TNF-α from macrophages in vitro
A cell suspension was distributed into each well of a 96-well plastic plate in a volume of 0·1 ml and incubated for 24 h in a 5% CO2 atmosphere incubator at 37°C. Supernatants were transferred to a plastic tube, centrifuged at 500 g and stored at −80°C until use for the TNF assay.
Preparation of supernatants from lung homogenates
After measuring lung weight, 0·2 g lung specimens were put into a tube containing 2 ml of serum-free RPMI-1640 medium supplemented with 50 µM 2-ME, 100 U/ml penicillin and 100 µg/ml streptomycin, and made homogenates by Physictron (Microtec Co., Ltd, Funabashi, Japan). After centrifugation at 5000 g for 30 min, supernatants were removed to estimate their TNF titres.
TNF assay
TNF levels were estimated by a cytotoxicity test using TNF-sensitive L929 (C5F6) cells (4 × 105/ml) in the presence of actinomycin D-mannitol (Sigma, St. Louis, MO, USA), as described previously [23]. Four hours before the termination of 20-h cultures, aliquots (10 µl) of WST-1 cell counting Kit (Dojindo, Kumamoto, Japan) were added to each well of 96-well microplates. Formazan produced by viable cells was determined by measuring absorbance at 450 nm (reference at 690 nm). The TNF level in the sample was calculated by multiplying the sample dilution with the value of recombinant murine TNF-α (Genzyme Inc., Boston, MA, USA) resulting in 50% cytotoxicity.
Anti-TNF-α monoclonal antibody (mAb)
An anti-TNF-α mAb clone (MP6-XT22) was incubated in serum-free SFM-101 medium in Teflon™ bags. The mAb in supernatants was concentrated by ultrafiltration using PM-10 (Millipore, Billerica, MA, USA), the 50% saturation with ammonium sulphate and then purified by a column chromatography using Hi-trap Protein-G (Amersham, Uppsala, Sweden). The specific activity of this mAb to give the 50% neutralization of TNF-α-induced cytotoxicity, estimated by the bioassay using TNF-sensitive L929 cells, was 5·27 mg/mg TNF-α. A potentially contaminated endotoxin level was 1·34 ng/mg mAb, estimated by the Limulus test. Anti-TNF-α mAb (0·5 mg) was given i.v. into IFN-γ–/– mice immediately before and on day 2 after immunization with 10 µg TDM.
Histology
Lung samples were removed from mice at different time-points after injections, fixed with 4% paraformaldehyde in 0·01 M phosphate buffer (pH 7·4) and embedded in paraffin. Sections were stained with haematoxylin and eosin. The sections were examined by light microscopy at a magnification of × 100 and × 400, and numbers of granulomas were counted for at least 20 fields. The number of granulomas per field were expressed as mean ± standard deviation (s.d.).
Statistics
The statistical significance of differences between groups was evaluated by Student's t-test. A P-value of less than 0·05 was taken as significant.
Results
TLR4-deleted B10Cr macrophages produced higher levels of TNF-α than B10Sn macrophages in response to TDM
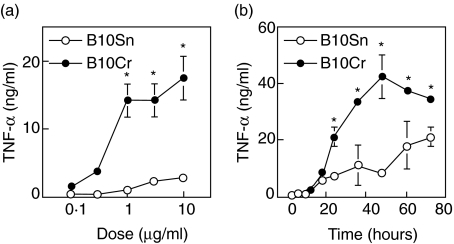
To answer the question of whether in vitro stimulation with very hydrophobic TDM triggers TNF-α production, peritoneal macrophages, obtained from the wild-type B10Sn and TLR4-defected and IL-12Rβ2-mutated B10Cr mice, were incubated for 24 h in wells coated with 0·1, 0·3, 1, 3 and 10 µg of TDM. Significant amounts of TNF-α were detected in culture supernatants of B10Cr macrophages in response to over 0·3 µg TDM per well. TNF levels in supernatants of B10Cr macrophage cultures were much higher than those in B10Sn macrophage cultures (Fig. 1a). When macrophages were stimulated with 10 µg TDM per well for the time indicated, significant TNF levels were detected from 18 h post-stimulation and afterwards. The TNF amounts in B10Cr macrophage cultures peaked at 48 h after stimulation (Fig. 1b). TNF production by B10Sn macrophages was lower than that in B10Cr cultures at each time-point, suggesting that signal transduction via TLR4/MD2 to produce TNF-α is not necessary for macrophage stimulation with TDM.
Fig. 1.
Dose- and time-dependent responses of peritoneal macrophages to trehalose dimycolate (TDM) coated on wells. Peritoneal macrophages were obtained from B10Sn and B10Cr mice injected with 2 ml of 10% proteose peptone 4 days previously. (a) Macrophages were stimulated with the indicated dose of TDM coated on wells for 24 h or (b) with 10 µg TDM for different time-points. Tumour necrosis factor (TNF)-α amounts were estimated by a bioassay using TNF-sensitive L929 cells. Results represent arithmetic mean ± s.d. of triplicate cultures in a representative experiment. *P< 0·05 by Student's t-test.
Comparison of TNF-α-producing activity between TDM, PIM and LAM using peritoneal and alveolar macrophages from B10Sn mice
To compare TNF-α-inducing activity between TDM, PIM and LAM, B10Sn macrophages were stimulated in vitro for 24 h with glycolipids coated on wells at a dose of either 1 or 10 µg per well. TDM had the strongest TNF-inducing activity compared with those of PIM and LAM (Table 1). Stimulation with PIM and LAM resulted in similar levels of TNF production in peritoneal macrophage cultures. In alveolar macrophage cultures, stimulation with LAM rather than PIM caused greater TNF-α production.
Table 1.
Comparison of tumour necrosis factor (TNF)-α production of mycobacterial glycolipids by macrophages obtained from B10Sn mice.
| TNF-α produced (pg/ml) | ||||
|---|---|---|---|---|
| Dose (µg/well) | TDM | PIM | LAM | LPS (0·1 µg/well) |
| Peritoneal macrophages | ||||
| 1 | 15733 ± 2507 | 1535 ± 629 | 1979 ± 86 | |
| 10 | 12465 ± 920 | 1918 ± 594 | 917 ± 154 | |
| Alveolar macrophages | ||||
| 1 | 7535 ± 2964 | 151 ± 69 | 1409 ± 417 | 18145 ± 2215 |
| 10 | 27778 ± 1387 | 2816 ± 669 | 9863 ± 405 | |
Peritoneal and alveolar macrophages (5 × 105/well) from B10Sn mice were stimulated with the indicated dose of test samples coated on wells for 24 h. TNF-α amounts in culture supernatants were estimated by a bioassay using TNF-sensitive L929 cells. Results represent arithmetic mean ± s.d. of triplicate cultures. Values of controls stimulated with medium were less than detectable. TDM: trehalose dimycolate, LAM: lipoarabinomannan; PIM: phosphatidylinositol mannosides; LPS: lipopolysaccharide.
Kinetics of pulmonary granuloma caused by TDM
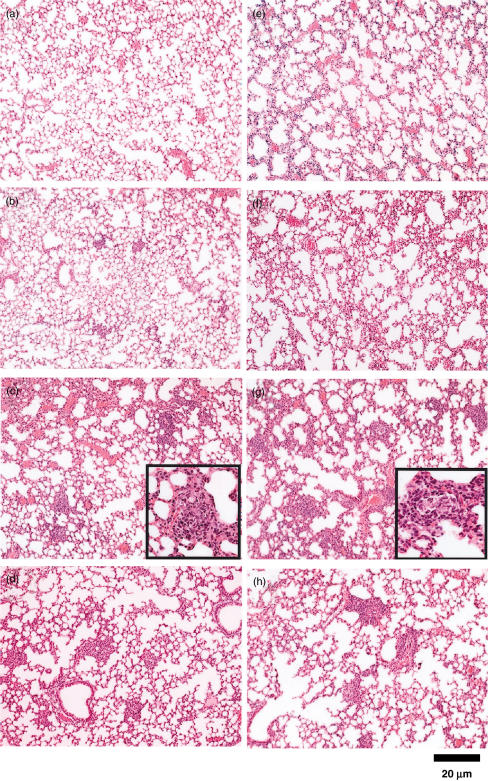
Because TDM exhibited the strongest TNF-inducing activity, kinetic study on pulmonary granuloma formation was compared between B10Cr and B10Sn mice injected i.v. with 10 µg TDM as the w/o/w emulsion. In B10Sn mice, very weak responses were detected on day 2 post-injection (Fig. 2b). On day 4, aggregates consisting of mixtures of lymphocytes and macrophages were observed widely at the medium size (Fig. 2c). On day 7, cell aggregates were decreased (Fig. 2d). In B10Cr mice, although the time-course of responses in lungs post-injection with TDM was similar to that in B10Sn mice (Fig. 2e–h), stronger inflammation than B10Sn mice and the presence of pulmonary epitheloid granulomas were observed on day 7 (Fig. 2g), suggesting that granuloma-inducing activity correlates closely with the TNF-inducing activity.
Fig. 2.
Granuloma formation induced by trehalose dimycolate (TDM) in the lungs of B10Sn and B10Cr mice. B10Sn (a–d) and B10Cr (e–h) mice were injected intravenously with 10 µg of TDM as the w/o/w emulsion via the tail vein. On days 0 (a, e), 2 (b, f), 4 (c, g) and 7 (d, h), pulmonary granuloma was observed microscopically. Photographs show a representative in a group of five mice. Enlarged photographs are shown in small tetragons of (c) and (g).
Comparison of pulmonary granuloma formation between TDM, PIM and LAM
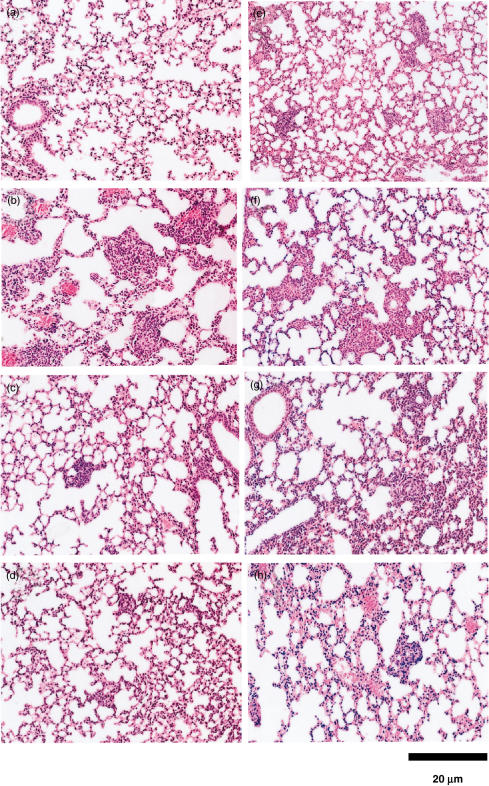
To compare granuloma-forming activity, B10Sn mice were injected i.v. with 10 µg of TDM, PIM or LAM as the w/o/w emulsion and the activity was estimated on day 7. As shown in Fig. 3a–d and Table 2, the strongest activity was observed in lungs of mice administered TDM. In addition to accumulation of endothelial cells, pulmonary granulomas developed clearly in TDM-administered mice. Although both LAM and PIM also had granuloma-forming activities, their activities were weaker than that of TDM. Numbers of granulomas correlated with TNF-α levels in alveolar macrophage cultures.
Fig. 3.
Comparison of granuloma-forming activity of TDM, PIM and LAM, and interferon (IFN)-γ-independent and tumour necrosis factor (TNF)-α-dependent granuloma formation. B10Sn mice were injected intravenously (i.v.) with 10 µg of TDM (b), PIM (c), LAM (d) as the w/o/w emulsion via the tail vein. Controls (vehicle alone) (a). BALB/c (e), BALB/lpsd (f) and IFN-γ–/– (g) mice were injected i.v. with 10 µg TDM. A group of IFN-γ–/– mice was treated twice with anti-TNF-α monoclonal antibody immediately before and on day 2 after TDM injection (h). Granuloma formation was analysed on day 7. Results show a representative of groups of five mice.
Table 2.
Comparison of granuloma-forming activity of trehalose dimycolate (TDM), lipoarabinomannan (LAM) and phosphatidylinositol mannosides (PIM) and interferon (IFN)-γ-independent and tumour necrosis factor (TNF)-α-dependent granuloma formation.
| Mice | Treated with mean ± s.d. | Number of granulomas | P-value |
|---|---|---|---|
| Experiment 1 | |||
| B10Sn | Vehicle | 1·0 ± 0·2 | |
| TDM | 6·3 ± 0·8 | 0·0003 | |
| PIM | 2·4 ± 0·3 | 0·0011 | |
| LAM | 3·6 ± 0·5 | 0·0012 | |
| Experiment 2 | |||
| BALB/c | TDM | 5·1 ± 2·3 | |
| IFN-γ−/− | TDM | 4·7 ± 0·8 | n.s. |
| IFN-γ−/− | anti-TNF-α mAb + TDM | 1·2 ± 0·5 | 0·043 |
Mice were injected intravenously with 10 µg of test samples or vehicle alone (controls) as the w/o/w emulsion via the tail vein, and granuloma formation was analysed on day 7. A group of interferon (IFN)-γ–/– mice was treated twice with anti-TNF-α monoclonal antibodies immediately before and on day 2 after TDM injection. Results show arithmetic means ± s.d. of five mice per group.
As it is possible that TNF-inducing activity relates to granuloma-forming activity, B10Sn mice were administered i.v. 10 µg of TDM, PIM and LAM, and bioactive TNF amounts in lung homogenates were determined by cytotoxicity test. TNF production peaked on day 2, declined quickly and returned to undetectable levels on day 4 (Fig. 4a). To compare TNF amounts in lungs, the TNF activity in homogenates was determined (Fig. 4b). Among them, TDM showed the strongest activity but not significant versus PIM.
Fig. 4.
Tumour necrosis factor (TNF)-α levels in the lung after intravenous injections with trehalose dimycolate (TDM), lipoarabinomannan (LAM) and phosphatidylinositol mannosides (PIM) as the w/o/w emulsion. (a) Kinetic study of TNF-α production in the lung after injection with 10 µg PIM. (b) Comparison of TNF-α levels in the lung among TDM, PIM and LAM. Control mice were injected with w/o/w emulsion without mycobacterial glycolipids. Results show arithmetic mean ± s.d. of three mice per group. *P< 0·05.
Pulmonary granuloma formation also occurred in IFN-γ–/– mice
To clarify whether or not pulmonary granuloma formation in a mouse model is IFN-γ-dependent, wild-type BALB/c and IFN-γ–/– mice were injected i.v. with 10 µg TDM (Fig. 3e–h and Table 2). In the wild-type BALB/c and tlr4-mutated BALB/lpsd mice, granuloma formation was confirmed (Fig. 3e,f). Furthermore, the presence of pulmonary granuloma was demonstrated clearly in IFN-γ–/– mice. To confirm the role of TNF-α for granuloma formation, anti-TNF-α mAb was administered twice to IFN-γ–/– mice immediately before and on day 2 after immunization with 10 µg TDM. Treatment with anti-TNF-α mAb resulted in a significant decrease of pulmonary granuloma formation (Fig. 3g,h and Table 2). These results indicate that pulmonary granuloma formation is IFN-γ-independent and TNF-α-dependent.
Discussion
The present study is the first report that PIM and LAM are also inducible granuloma formation as well as TDM. We demonstrated clearly that pulmonary granuloma formation due to administration of mycobacterial glycolipids TDM, LAM and PIM as the w/o/w emulsion was IFN-γ- and TLR4-independent (Figs 2 and 3). These glycolipids stimulated mouse macrophages via at least TLR2 molecules to activate NF-κB and to produce cytokines such as TNF-α and IL-1β (data not shown). Furthermore, in vitro TNF-inducing activity of mycobacterial glycolipids appeared to correlate with in vivo granuloma-forming activity in mice, suggesting that TNF-α plays an important role for granuloma formation. This coincided well with the results of Perez et al. [14], that production of inflammatory cytokines TNF-α, IL-1β and IL-6 related closely to pulmonary granuloma formation in murine lung. In human tuberculosis, however, the development of chronic pulmonary granulomas with caseous necrosis depends on the activation of Th1 cells capable of producing IFN-γ. It has been considered that TDM is responsible for the pathogenesis of pulmonary granuloma formation. In mice, pulmonary granulomas caused by mycobacterial glycolipids are of transient inflammation, but not chronic [24]. There is a large difference between human and murine systems regarding presentation of lipid antigens to T cells by CD1 molecules, composed of transmembrane major histocompatibility complex (MHC) class I-like molecules and β2 microglobulin. They are separated into two groups, group I (CD1a, CD1b, CD1c and CD1e) and group II (CD1d). Both groups are present in humans, while CD1d is the only isoform expressed in murine rodents. In the human system, foreign mycobacterial glycolipids are presented by CD1a, CD1b and CD1c molecules to T cells [1,2,11,25]. In contrast, it is likely that human and murine CD1d-restricted T cells recognize self-lipid antigens in vivo and α-galactosylceramide in experimental systems [26–31]. Therefore, it seems that the difference in the presentation of foreign lipid antigen to T cells results in different responses in pulmonary granuloma formation. We have recently established murine T cell clones reactive to mycobacterial glycolipids such as PIM and LAM, and obtained several hybridomas capable of producing monoclonal IgG antibodies against the lipid part of LAM and PIM (unpublished data), indicating that even in mice mycobacterial glycolipids are presented to helper T cells due to unknown mechanisms. Although the possibility still remained that IFN-γ produced by Th1 cells contributes to pulmonary granuloma formation, the present study showed that pulmonary granulomas were present in not only B10Cr mice lacking signalling via IL-12R, but also IFN-γ–/– mice (Fig. 3), indicating that the activation of Th1 cells and production of IFN-γ do not occur in these mouse strains. Thus the contribution of Th1 cells and their product IFN-γ to pulmonary granuloma formation in mice due to mycobacterial glycolipids seems to be negligible.
There is a distinct difference between TDM and LAM in terms of their chemical structure and hydrophobic nature. TDM consists of two mycolic acids (C80 to C82) bound to the OH groups at the C6 and C6′ positions of trehalose. PIM, the lipid part of LAM, carries three or four molecules of palmitic (C16) and tuberculostearic (C19) acids bound to the OH groups of not only glycerol backbone but also myo-inositol and mannoside residues. As the arabinomannan bound to the mannose residue of PIM is the constituent of LAM, LAM is much hydrophilic than TDM and soluble in water. To compare in vitro the activity between extremely hydrophobic TDM and relatively hydrophilic LAM, it should be performed under similar conditions. Because the conditions would be different if TDM were suspended in the medium, we therefore coated glycolipids on the surface of plastic plates to make the conditions as similar as possible. It is likely that the carbon chain length of the acyl group reflects on granuloma formation in lungs of mice when they are given as w/o/w emulsions. Indeed, the activity of TDM consisting of mycolate (C80 to C82) was stronger than that of PIM and LAM consisting of palmitoyl and tuberculostearoyl groups (Fig. 4).
In conclusion, mycobacterial glycolipid-induced pulmonary granuloma formation in mice is caused by IFN-γ-independent and TNF-α-dependent mechanisms.
Acknowledgments
We would like to thank Ms Akiko Kato and Ms Rie Asako for technical assistance. This study was supported in part by the Grant of the 21st Century COE Program, Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to YK) and the Human Science Foundation (KH31031 to YK).
References
- 1.Behling CA, Bennett B, Takayama K, Hunter RL. Development of a trehalose 6,6′-dimycolate model which explains cord formation by Mycobacterium tuberculosis. Infect Immun. 1993;61:2296–303. doi: 10.1128/iai.61.6.2296-2303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsunaga I, Oka S, Fujiwara N, Yano I. Relationship between induction of macrophage chemotactic factors and formation of granulomas caused by mycoloyl glycolipids from Rhodococcus ruber (Nocardia rubra) J Biochem (Tokyo) 1996;120:663–70. doi: 10.1093/oxfordjournals.jbchem.a021463. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi I, Ikeda N, Nakayama M, Kato Y, Yano I, Kaneda K. Trehalose 6,6′-dimycolate (Cord factor) enhances neovascularization through vascular endothelial growth factor production by neutrophils and macrophage. Infect Immun. 2000;68:2043–52. doi: 10.1128/iai.68.4.2043-2052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grand-Perret T, Lepoivre M, Petit JF, Lemaire G. Macrophage activation by trehalose dimycolate requirement for an expression signal in vitro for antitumoral activity; biochemical markers distinguishing primed and fully activated macrophage. Eur J Immunol. 1986;16:332–8. doi: 10.1002/eji.1830160403. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe R, Yoo YC, Hata K, et al. Inhibitory effect of trehalose dimycolate (TDM) and its stereoisometric derivatives, trehalose dicorynomycolates (TDCMs), with low toxicity on lung metastasis of tumour cells in mice. Vaccine. 1999;17:1484–92. doi: 10.1016/s0264-410x(98)00367-3. [DOI] [PubMed] [Google Scholar]
- 6.Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68:5991–7. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamasaki N, Isowa K, Kamada K, et al. In vivo administration of mycobacterial cord factor (trehalose 6, 6′-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect Immun. 2000;68:3704–9. doi: 10.1128/iai.68.6.3704-3709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K, Hasunuma R, Horikoshi T, et al. Induction of hypersensitivity to endotoxin lethality in mice by treatment with trehalose 6,6′-dimycolate but not with 2,3,6,6′-tetaacyl trehalose 2′-sulfate. J Endotoxin Res. 1999;5:23–30. [Google Scholar]
- 9.Ryll R, Watanabe K, Fujiwara N, et al. Mycobacterial cord factor, but not sulfolipid, causes depletion of NKT cells and upregulation of CD1d1 on murine macrophages. Microbes Infect. 2001;3:611–9. doi: 10.1016/s1286-4579(01)01416-2. [DOI] [PubMed] [Google Scholar]
- 10.Oswald IP, Dozois CM, Petit JF, Lemaire G. Interleukin-12 synthesis is a required step in trehalose dimycolate-induced activation of mouse peritoneal macrophages. Infect Immun. 1997;65:1364–9. doi: 10.1128/iai.65.4.1364-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oswald IP, Dozois CM, Fournout S, Petit JF, Lemaire G. Tumor necrosis factor is required for the priming of peritoneal macrophages by trehalose dimycolate. Eur Cytokine Netw. 1999;10:533–40. [PubMed] [Google Scholar]
- 12.Silva CL, Tincani I, Brandao Filho SL, Faccioli LH. Mouse cachexia induced by trehalose dimycolate from Nocardia asteroids. J Gen Microbiol. 1988;134:1629–33. doi: 10.1099/00221287-134-6-1629. [DOI] [PubMed] [Google Scholar]
- 13.Wallias RS, Amir-Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–52. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez RL, Roman J, Roser S, et al. Cytokine message and protein expression during lung granuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6′-dimycolate. J Interferon Cytokine Res. 2000;20:795–804. doi: 10.1089/10799900050151067. [DOI] [PubMed] [Google Scholar]
- 15.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 19.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 20.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan P, Ballou CE. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. The family of dimannophosphoinositides. J Biol Chem. 1967;242:3046–56. [PubMed] [Google Scholar]
- 22.Yano I, Tomiyasu I, Kaneda K, et al. Isolation of mycolic acid-containing glycolipids in Nocardia rubra and their granuloma forming activity in mice. J Pharmacobiodyn. 1987;10:113–23. doi: 10.1248/bpb1978.10.113. [DOI] [PubMed] [Google Scholar]
- 23.Hasunuma R, Morita H, Tanaka S, et al. Differential clearance and induction of host responses by various administered or released lipopolysaccharides. J Endotoxin Res. 2001;7:421–9. [PubMed] [Google Scholar]
- 24.Algood HMS, Chan J, Fynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 2003;14:467–77. doi: 10.1016/s1359-6101(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 25.Beckman EM, Porcelli A, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 26.Brossay L, Chioda M, Burdin N, et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnaud C, Lee D, Donnars O, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 28.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moody DB, Reinhold BB, Guy MR, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–6. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 30.Rosat JP, Grant EP, Beckman EM, et al. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+αβ T cell pool. J Immunol. 1999;162:366–71. [PubMed] [Google Scholar]
- 31.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with α-galactosylceramide. J Immunol. 1999;163:2387–91. [PubMed] [Google Scholar]