Figure 4. Control of catalytic activity by trans cyclic interactions of the C-terminal tail.
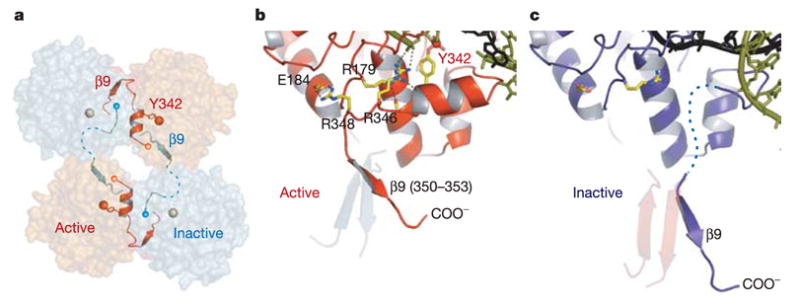
a, The assembly of active (red) and inactive (cyan) catalytic sites results from a skewed packing arrangement of λ -int(75–356) subunits in the tetramer. The scissile phosphates bound by active and inactive subunits are shown as red and grey spheres, respectively. b, In the active conformation, the tail is oriented to permit stabilizing interactions with residues Arg 346 and Arg 348 that result in a well-ordered segment (residues 339–348) containing the Tyr 342 nucleophile. c, In the inactive molecule, a large shift of β 9 causes this segment to become disordered.
