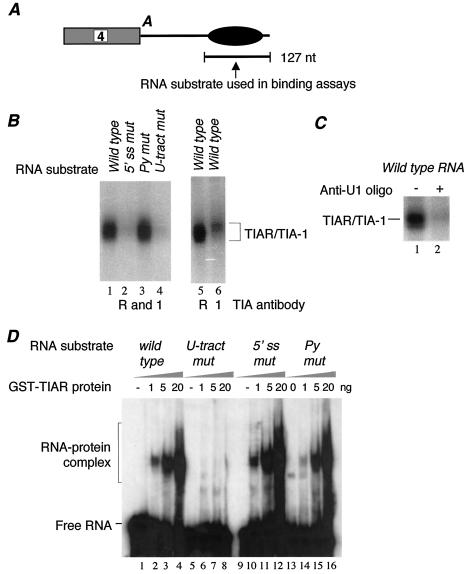
FIG. 2.
TIAR specifically interacts with the U tract in the enhancer element. (A) Diagram showing the RNA substrate used in UV cross-linking and gel mobility shift assays. A, polyadenylation signal. (B) Interaction of TIAR and TIA-1 with the intron enhancer element in HeLa nuclear extract depends on both the U tract and the pseudo 5′ splice site sequence. The 32P-labeled in vitro-transcribed RNA containing either wild-type or mutated enhancer sequence was UV cross-linked in HeLa cell nuclear extract and immunoprecipitated with antibodies specific to both TIAR and TIA-1 (R and 1; lanes 1 to 4), TIAR (R; lane 5), or TIA-1 (1; lane 6). Wild-type intron enhancer (lanes 1, 5, and 6) and enhancers in which either the pseudo 5′ splice site (5′ ss mut; lane 2), pyrimidine (Py mut; lane 3), or U tract (U-tract mut; lane 4) sequence was mutated (Fig. 1C) were used in this assay. (C) Interaction of TIAR with the intron enhancer element depends on the 5′ end of U1 snRNA. UV cross-linking-IP assays were carried out with the wild-type enhancer in the absence (lane 1) or presence (lane 2) of a 2′-O-methyl ribo-oligonucleotide complementary to the 5′ terminus of U1 RNA. (D) Recombinant GST-TIAR binds to the U tract sequence specifically. Gel shift analysis was carried out with increasing amounts of GST-TIAR and 32P-labeled RNA containing the wild-type enhancer (lanes 1 to 4) and enhancers in which the U tract (lanes 5 to 8), the pseudo 5′ splice site (lanes 9 to 12), or the pyrimidine (lanes 13 to 16) sequence was mutated.