Abstract
Purpose
A rare, familial early-onset form of Fuchs corneal dystrophy (FCD) is caused by mutation in the COL8A2 gene. This study describes the aberrant pattern of distribution of collagen type VIII and basement membrane components in Descemet’s membrane (DM) and endothelium of three individuals with the same L450W mutation that represent different stages of early-onset FCD.
Methods
Immunohistochemical studies with bright field, fluorescence, and confocal microscopy characterized the pathology of sectioned corneal buttons with antibodies against COL8A1, COL8A2, COL4, laminin, and fibronectin. A portion of each was processed for electron microscopy.
Results
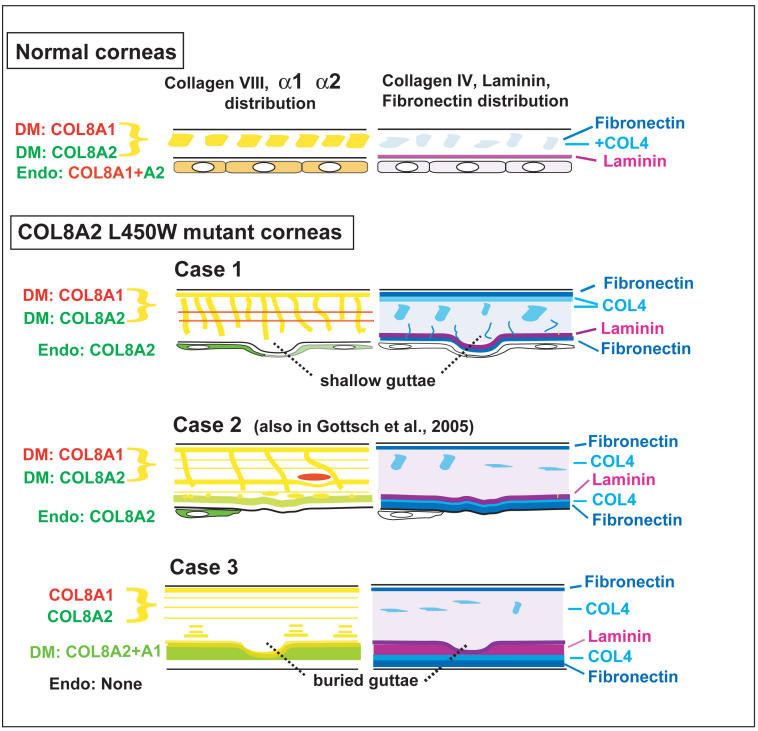
Histologic examination of pathologic changes in case 1 demonstrated relative preservation of the endothelium, whereas in case 2 much of this layer was atrophic and in case 3 there was complete loss of the endothelium. DM also increased in thickness to 25 μm for case 1, to 31 μm for case 2, and to 38 μm for case 3. Case 1 was the only specimen to reveal shallow warts along the posterior surface of DM, whereas the most advanced specimen, case 3, showed evidence of earlier nodularity that had been buried by the accretion of further extracellular matrix material. The posterior aspect of DM in this specimen had the unusual property of lighter staining relative to the anterior region of DM, laid down earlier in life. Immunocytochemistry revealed increased expression and complex, sharply defined patterns of deposition of collagen VIII, collagen IV, laminin, and fibronectin. Ultrastructurally, the posterior nonbanded layer of DM was intermixed with banded collagen, and the posterior region of DM showed a high density of foci of spindle-shaped structures with intense-staining bands, spaced at ~ 120 nm. Finally, ultrastructural studies of the endothelium in case 1 revealed unusual accumulation of swelling mitochondria. The endothelial cells also had large amounts of abnormal prominent rough endoplasmic reticulum. Type VIII collagen alpha 2 immunogold signal was associated with the highly granular ribosomes of the rough endoplasmic reticulum of these patients.
Conclusions
Microscopic and electron microscopic examination revealed pathological changes in DM of L450W COL8A2 mutants that were consistent with several-fold increased growth of the extracellular matrix and progressive deposition and synthesis of extracellular material by endothelial cells. As with late-onset FCD, this is accompanied by attenuation and eventual loss of the endothelium itself. Whether the abnormal deposition of collagen, laminin, and fibronectin contributes to the dysfunction and death of the endothelium remains to be determined.
INTRODUCTION
Fuchs corneal dystrophy (FCD) is characterized by the progressive degeneration of corneal endothelial cells, which leads to corneal decompensation. There are two distinct forms of FCD: an extremely rare early-onset form1 and a late-onset form, which affects 4% of the general population over age 40. Early-onset FCD is inherited with high penetrance and associated with dominant mis-sense mutations in the COL8A2 gene at chromosome 1p34.3.1,2 Recently, two genetic loci for dominantly inherited late-onset FCD have been mapped to chromosome 133 and chromosome 18,4 although the genes at these loci are not yet known.
The authors of this study have previously reported an immunopathological study of one case of early-onset FCD with a COL8A2 mutation and compared its histology with normal corneas and with late-onset FCD.5 Both early- and late-onset forms of FCD showed thickening of Descemet’s membrane (DM), attenuation of corneal endothelial cells, and aberrant, massive deposits of both α and β isoforms of collagen VIII. FCD caused by the COL8A2 L450W mutation showed much greater thickening of DM and appeared to completely lack the highly distinctive guttae, excrescences of extracellular matrix that typically protrude from the posterior face of DM.
Several decades ago, Magovern6 did a detailed histopathological study on the corneal buttons of the family members of early-onset FCD and identified three main types of histological changes of DM in these patients. With the advancement of gene mapping and mutation analysis, we now know that the genes responsible for early-onset and late-onset FCD are most likely different. So far no detailed study of immunopathological characterization has been reported on patient-to-patient variation in the histological changes in COL8A2 early-onset FCD.
This study investigates corneal buttons excised during transplant surgery from three rare early-onset FCD cases, all obtained from the same family, and with the same COL8A2 mutation. These three patients appear to be in different stages of the disease process, as determined by clinical course and the degree of preservation of the corneal endothelium. Histochemical techniques, including light and electron microscopy, are used to examine collagen VIII and other basement membrane components in order to better understand the underlying pathology and its variability. This study has the special advantage of examining FCD caused by the same gene mutation and therefore avoids the problems associated with studies of a heterogeneous disorder of unknown origin.
METHODS
PATIENTS
The study protocol was approved by the Joint Committee on Clinical Investigation at the Johns Hopkins University School of Medicine and was in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study participants. Patients were recruited for study following initial evaluations of patients with FCD who presented to the Cornea Service at the Wilmer Ophthalmological Institute. Patient recruitment and participation followed institutional review board approved procedures.
IMMUNOHISTOCHEMISTRY
For immunohistochemical study, freshly excised corneal buttons were marked superiorly to preserve orientation, bisected vertically in the central region, and one half immediately immersed in fresh 4% paraformaldehyde–phosphate-buffered saline (PBS), fixed overnight at 4°C, transferred to 20% sucrose, embedded in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Tokyo, Japan), and frozen in a mixture of methyl butane and dry ice. Blocks were cryosectioned at 9-μm thickness.
The antibodies used for this study and their dilutions were described previously,5 including type IV, type VIII alpha 1 and 2 collagen, fibronectin, and laminin antibodies. After the sections were dried at room temperature for 10 minutes, they were successively incubated with blocking serum and mouse monoclonal or rabbit primary antibodies overnight at 4°C and then anti-mouse or anti-rabbit secondary antibody conjugated with cy-3 (1:100; Jackson ImmunoResearch, West Grove, Pennsylvania). For double labeling, the sections were incubated overnight with two primary antibodies from different species and then the secondary fluorescent antibodies labeled with either cy-2 or cy-3 (Jackson ImmunoResearch). Slides were stained with Hoechst (1:2000; Molecular Probes, Eugene, Oregon) for 60 seconds and mounted with fluorescent mounting medium. Specimens were viewed with a fluorescence microscope and a confocal microscope (Ultraview 2; Perkin Elmer, Boston, Massachusetts).
STANDARD ELECTRON MICROSCOPY
For ultrastructural studies, a portion of each corneal button was fixed in 1% glutaraldehyde and 4% formaldehyde, then postfixed with osmium tetroxide. Plastic 1.2-μm-thick sections were stained with p-phenylenediamine and viewed by light microscopy (Figure 1). Ultrathin sections were stained with tannic acid and lead citrate, then viewed by transmission electron microscopy.
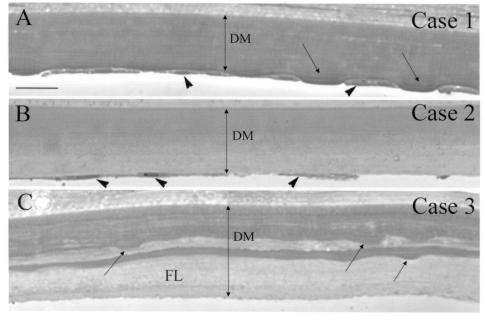
FIGURE 1.
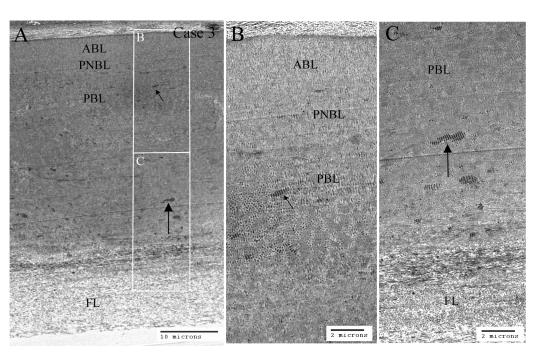
Light microscopy of Descemet’s membrane (DM) in early-onset Fuchs corneal dystrophy (FCD). Plastic sections were stained with p-phenylenediamine to reveal compositionally distinct mixtures of extracellular matrix in DM. All three cases showed marked thickening of DM. A, Case 1 (least advanced FCD). Multiple shallow guttae (arrows) in the posterior surface, with flattened endothelial cells (arrowheads) between the guttae. Uniform staining of DM. B, Case 2 (more advanced FCD). Fewer remaining endothelial cells (arrowheads). Dark staining of anterior half of DM and slightly lighter staining in posterior half. No guttae were noted in this specimen. C, Case 3 (most advanced FCD). DM was thicker than in case 1 and case 2 and showed laminar interdigitation of dark and very lightly stained regions. Sections revealed buried guttae (arrows) covered by a lightly stained fibrillar layer (FL). All endothelial cells were missing from the posterior face of DM. Bar = 20 μm.
IMMUNOELECTRON MICROSCOPY
Small pieces of 4% paraformaldehyde-fixed corneal tissue were postfixed in 1% osmium tetroxide for 1 hour. The tissues were dehydrated through series of graded alcohol, then were embedded in L.R. White embedding medium (Ted Pella, Redding, California) with anaerobic polymerization and heated at 65°C for 2 days. Ultrathin sections were cut at 60-nm thickness. Sections were collected on formvar-carbon nickel grids. Staining procedure was similar to the regular immunohistochemistry. Briefly, the grids were floated onto 20-μL droplets of the following solutions: PBS, donkey serum, primary antibody (rabbit COL8A2 antibody, 1:1500) overnight at 4°C and then 12-nm colloidal gold-conjugated secondary antibody (Donkey anti-rabbit; Jackson ImmunoRes, 1:30) for 1 hour at room temperature. The grids were finally washed in double-distilled water three times and observed under transmission electron miscroscopy.
RESULTS
LIGHT MICROSCOPY
Thick plastic sections were used for the light microscopy (Figure 1), which revealed thicker-than-normal DM for each of the three specimens: case 1 was 25 μm, case 2 was 31 μm, and case 3 was 38 μm. The specimens of each case appear to follow a clear progression of severity. Case 1 appeared to represent a relatively early stage of the disease process, with the thinnest DM of the three, and still retained substantial endothelial cell coverage. In much of the section, shallow guttae were visible on the posterior face of DM. Endothelial cells tended to flank the guttae and were significantly flattened. In case 2, DM was slightly thicker and no obvious guttae were visible. This represents a previously published case5 and constitutes a more advanced case, as fewer endothelial cells were seen. The DM showed a fibrous posterior layer adjoining the endothelial cells. This posterior DM region stained more lightly, suggesting subtle differences in protein density or content. Case 3 represented the most advanced (or severe) case of early-onset FCD, since nearly all the endothelial cells were absent. The DM was also thicker than in either case 1 or case 2. Additionally, DM had pronounced posterior and interstitial lamination with more lightly stained extracellular matrix. Guttae-like structures (buried warts) were visible inside the DM and were covered posteriorly by the lightly stained loose fibrillar layer of matrix protein.
IMMUNOHISTOCHEMISTRY OF COL8A2 L450W FCD
In case 1, the least advanced of the three cases, COL8A1 and COL8A2 staining revealed abundant, intensely labeled vertical fibers spanning DM with an immunopositive anterior fetal layer (Figure 2). Double labeling showed that the two collagen VIII isoforms were largely, but not completely, colocalized. In case 2, the DM displayed a more heterogeneous labeling pattern with COL8A1 and COL8A2 antibodies (Figure 3). COL8A1 and COL8A2 both displayed a fiber-like labeling inside the DM, very similar to case 1 (Figure 3, D through F). Other areas in the specimen showed no fibrous structures and, instead, punctate or band-like staining (Figure 3, G through L).
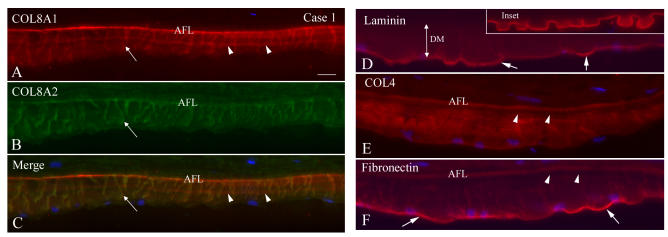
FIGURE 2.
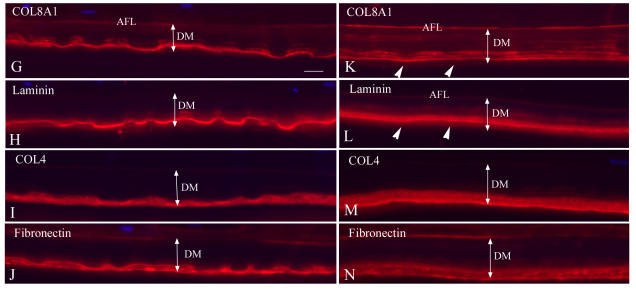
Single and double immunolabeling with various antibodies in case 1 of early-onset Fuchs corneal dystrophy (FCD). A through C, Both COL8A1 and COL8A2 antibodies showed intensely labeled anterior fetal layer (AFL) and thick vertical fibers (arrows) spanning the Descemet’s membrane (DM) and mostly colocalized. COL8A1 labeling also showed a middle layer of positive staining (arrowheads). D, Laminin staining showed intense linear pattern that defined the contour of guttae. Note the shallow guttae (arrows) in this early-onset FCD compared to the classic guttae in late-onset FCD (inset). E, COL4 labeling showed more diffuse positive staining throughout the DM. An empty gap (arrowheads) was noted posterior to the positive AFL layer, which may represent posterior nonbanded layer. F, Fibronectin showed intense staining on the posterior surface of the DM (arrows) and positive wisp-like pattern staining radiating from the posterior surface of DM. Again, the empty gap was seen posterior to the AFL (arrowheads). Bar = 20 μm.
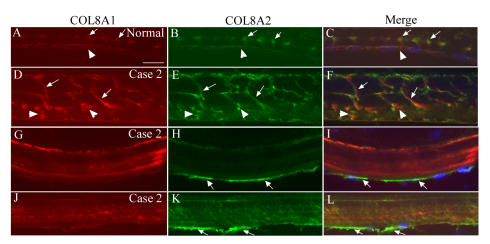
FIGURE 3.
COL8A1 and COL8A2 double labeling in the endothelial cells of normal and early-onset Fuchs corneal dystrophy (FCD) in case 2 (confocal imaging). A through C, In normal cornea, endothelial cells showed weakly positive staining with both COL8A1 and COL8A2 antibodies (arrowheads). Note the positive array of labeling in the anterior banded layer of Descemet’s membrane (DM) (arrows). D through F, COL8A1 and COL8A2 labeling showed intense positive vertically arranged serpiginous structures traversing the middle of DM and mostly colocalized (arrows). In the posterior DM, some scatter punctate staining with both antibodies was seen and partially colocalized (arrowheads). G through L, In early-onset FCD, endothelial cells showed weak staining with COL8A1 antibody, but intense signal with COL8A2 antibody (arrows). Note COL8A1 and COL8A2 labeling pattern in the DM was different than in D through F. Bar = 20 μm.
In case 3 (Figure 4), in which FCD was most advanced, the COL8A1 and COL8A2 positive signal was again seen in the posterior segment of DM as a strongly stained band pattern. The two collagen VIII isoforms were again mostly colocalized, but COL8A2 gave the strongest signal in the posterior DM (Figure 4, A through C). Focal areas of band-like or discrete staining were again visible inside the DM. In case 1 and case 2, some endothelial cells exhibited more intense staining with COL8A2 antibody than is visible in normal control endothelial cells (Figure 3). This suggests that in corneas with the COL8A2 L450W mutation, endothelial cells may have more active COL8A2 protein synthesis. This could explain the apparent high concentration of collagen VIII deposited in the posterior edge of DM during later stages of the disease.
FIGURE 4.
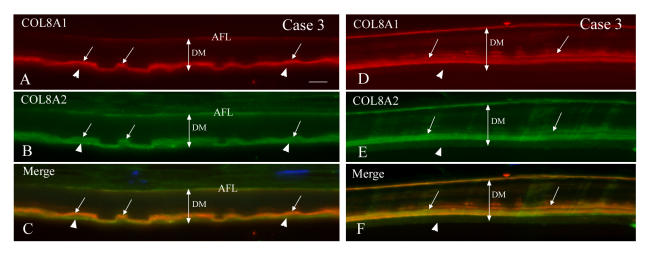
Single and double immunofluorescent labeling with COL8A1, COL8A2, COL4, laminin and fibronectin antibodies in case 3 of early-onset Fuchs corneal dystrophy.
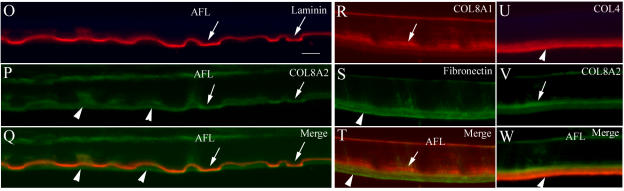
Panel A through F: Double immunolabeling with COL8A1 and COL8A2 antibodies in two different regions of Descemet’s membrane (DM) (case 3). A through C, Area with guttae. COL8A1 and COL8A2 were both located in the anterior fetal layer (AFL) and posterior segment of DM and mostly colocalized (arrows). In some areas, COL8A2 appeared to be on the posterior surface without COL8A1 staining (arrowheads). D through F, Area without guttae. COL8A1 and COL8A2 were colocalized in the posterior segment of DM as a band-like pattern (arrows) and some discrete staining in the middle of DM. However, the far posterior layer showed negative staining with both antibodies (arrowheads). Bar = 20 μm.
Panel G through N: Single immunolabeling with various antibodies on adjacent sections (case 3). G through J, Area with guttae. COL8A1 and laminin antibodies showed curved linear pattern staining, which was comparable to the posterior surface of the guttae in the middle layer of the posterior segment of DM. Some discrete staining was also seen inside DM with COL8A1 antibody. COL4 and fibronectin labeling showed positive signal in the far posterior surface of DM as a band-like pattern to cover the guttae. Note the flat posterior surface of DM facing the anterior chamber due to the collagen type IV and fibronectin components. K through N, Area without guttae. COL8A1 showed more intense labeling in the AFL and middle layer of posterior segment of DM. Laminin also showed similar pattern as COL8A1 staining, but no significant staining in the AFL. Note the far posterior layer of the DM was negative with these two antibodies (arrowheads). However, COL4 and fibronectin labeled the posterior stratum of the DM as a band-like pattern. Bar = 20 μm.
Panel O through W: Double labeling of laminin with COL8A2, COL8A1 with fibronectin, and COL4 with COL8A2 antibodies (case 3). O through Q, Laminin and COL8A2 antibodies in the area with guttae showed curved profile staining (mostly colocalized, arrows), which correlates to the posterior surface of the guttae in the anterior portion of the posterior region of DM. Some areas of posterior DM were only positive with COL8A2 antibody (arrowheads). Note laminin was negative in AFL layer. R through T, The posterior layer of DM stained positively only with fibronectin antibody (green, arrowheads), not with COL8A1 staining (red, arrows). COL8A1 labeling (arrows) was located anterior to the fibronectin positive staining. U through W, COL4 (red) signal was most intense in the posterior surface layer of DM (arrowheads), whereas the COL8A2 (green) signal was most intense in the middle layer of the posterior segment of DM (arrow), partially colocalized with COL4 staining as indicated by the yellow color of the junctional areas. Bar = 20 μm.
For the three cases, other extracellular matrix components of DM generally showed similar, but in some cases distinct, anomalies. In case 1, the collagen type IV, laminin, and fibronectin labeling showed intense signal in the posterior surface of DM (Figure 2), especially in the posterior surface of guttae. Laminin showed clear imaging of shallow guttae, which differ from the classic guttae observed in late-onset FCD (inset, Figure 2D). COL4 immunostaining showed more diffuse labeling in DM, and fibronectin also showed a faint wisp-like pattern radiating from the posterior surface of DM, comparable to the collagen VIII–containing structures. In case 3, buried guttae were covered by a loose fibrillar layer as shown in light microscopic plastic sections. With double labeling using various antibodies, this loose fibrillar band-like layer showed intense staining with collage IV and fibronectin antibodies (Figure 4, R through W). Laminin was located at the junction of collagen VIII with collagen IV and fibronectin (posterior border of the buried warts) and mixed with COL8A2 labeling in some areas (Figure 4, O through Q). All of these immunocytochemical differences are summarized in Figure 5.
FIGURE 5.
Diagram summarizing differences in distribution of collagen VIII isoforms (left panels) and collagen IV, laminin, and fibronectin (right panels) in normal Descemet’s membrane and endothelium, and the corresponding features in cases 1, 2, and 3 of early-onset Fuchs corneal dystrophy. Yellow signal in left panels represents colocalization of roughly equal immunocytochemical signal for COL8A1 and COL8A2. Yellow-green indicates predominance of COL8A2, and green indicates only COL8A2 signal.
ELECTRON AND IMMUNOELECTRON MICROSCOPY
Electron microscopic study of the conventionally stained specimens from case 1 and case 3 showed large amounts of disorganized banded collagens and wide-space collagens throughout DM (Figures 6 and 7). In case 1, wide-space collagen, appearing as atypical, irregularly formed structures, was present in the posterior DM, just anterior to the degenerated endothelial cells (Figure 7C, arrows). There were multiple shallow guttae in the posterior DM, which showed an abundant accumulation of wide-space collagen in the guttae (Figure 8, arrows). In case 3, in the posterior nonbanded layer, nonbanded material was intermixed with banded collagen (Figure 6B). Spindle-shaped wide-space collagen, typical of late-onset FCD, was found in the posterior banded layer (Figure 6, B and C). The posterior lightly staining extracellular matrix was composed primarily of collagen fibrils, with no banded collagens visible (Figure 6C).
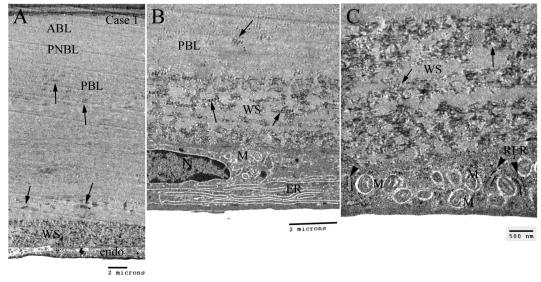
FIGURE 6.
Transmission electron microscopic study of Descemet’s membrane (DM) in early-onset Fuchs corneal dystropohy of case 3. A, Lower magnification showing the full-thickness of the DM. B and C, high magnification of the DM. The pictures showed typical four layers of the electron microscopic structure: anterior banded layer (ABL), posterior nonbanded layer (PNBL), posterior banded layer (PBL), and fibrillar layer (FL). PNBL was intermixed with banded collagens, and PBL was markedly thickened and contained numerous foci of wide-space collagen (small and large arrows). The posterior FL of DM was composed of abundant fibrillar basement membrane materials.
FIGURE 7.
Transmission electron microscopy showing Descemet’s membrane (DM) and endothelial cell in case 1 of early-onset Fuchs corneal dystrophy. A, Low magnification showing the full-thickness of the DM. There were many foci of wide-space collagen (arrows) in the posterior banded layer (PBL). Excessive amount of wide-space (WS) collagen were noted anterior to the endothelial cell. B, Higher magnification showing the WS collagen and an endothelial cell exhibiting abundant prominent endoplasmic reticulum (ER) and some degenerated mitochondria (M). C, Higher magnification showing the poorly formed WS collagen (arrows) and rough ER (arrowheads) and unusually abundant degenerated mitochondria (M) in the endothelial cell. ABL = anterior banded layer; PNBL = posterior nonbanded layer.
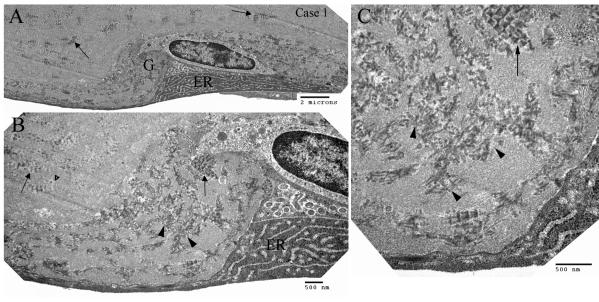
FIGURE 8.
Transmission electron microscopy showing ultrastructure of a shallow guttae in case 1 of early-onset Fuchs corneal dystrophy at different magnification. The guttae (G) contained abundant poorly formed (arrowheads) and a few well-formed (arrows) wide-space collagens. The endothelial cell was bordered by wide-space collagens and showed abundant prominent rough endoplasmic reticulum (ER) and degenerated mitochondria.
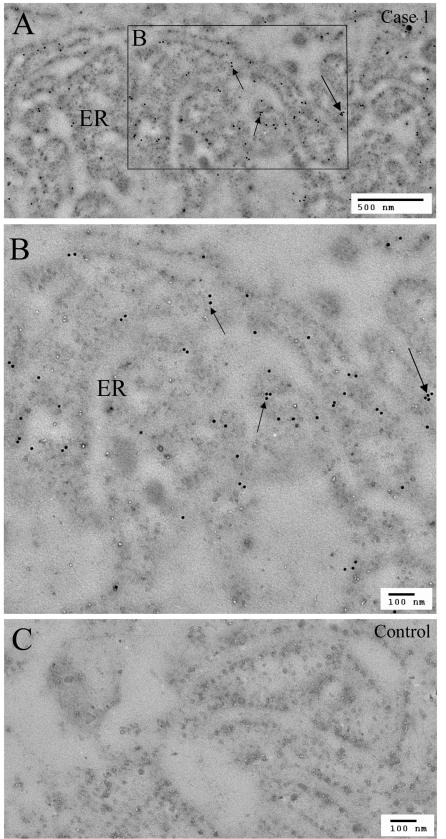
Immunoelectron microscopy with COL8A2-specific antibody was carried out to further characterize the location of this protein in the endothelial cells. The control section without primary antibody showed a low background signal (Figure 9C), whereas early-onset FCD showed strong colloidal gold labeling for COL8A2 in the ribosomal layer of the rough endoplasmic reticulum (RER), with less signal within the lumen of the RER (Figure 9, A and B). In case 3, the posterior loose fibrillar layer showed no COL8A2 immunogold labeling (not shown).
FIGURE 9.
Immunogold labeling of COL8A2 in the rough endoplasmic reticulum (ER) of the endothelial cell in case 1 of early-onset FCD. Abundant rough ER in the endothelial cell showed many gold-labeling particles on the ER membrane structures (arrows). A, low magnification; B, high magnification; C, negative control in a similar area of adjacent section.
DISCUSSION
In individuals carrying the dominant, fully penetrant L450W COL8A2 mutation, the disease process may be evident in early childhood. Endothelial dysfunction and corneal decompensation develop by the third to fourth decade of life,2 in contrast to late-onset FCD, which starts at middle age and develops to an advanced stage by the sixth to ninth decade. When observed clinically by retroillumination or specular confocal microscopy, this early-onset disease has a distinctive appearance, with unusual “shallow guttae.” In this study, we have identified these guttae histologically, and through the phenotypic variation found within this family, we have gained insight into the nature of these “shallow guttae” and why they were not observed in our earlier study.5 The three cases reported here are comparable to the different types of DM histopathology reported earlier by Magovern and associates6 in characterizing corneas of the same family. They described three main forms using light and electron microscopy: (1) pure corneal guttae, (2) DM of unusual thickness but without guttae, and (3) guttae buried by subsequent layers of more posterior extracellular matrix. In this previous report, the guttae and their appearance were interpreted as classic FCD morphology, as observed in late-onset FCD by Hogan and colleagues7 and Waring and associates.8
From the current study, however, it is clear that the guttae in L450W COL8A2 patients are structurally and developmentally different from those observed in late-onset FCD. When found on the posterior surface, they had a much flatter appearance. Usually, however, they took the form of structural discontinuities buried deep within DM. In the L450W COL8A2 mutant cornea, the thickness of DM was in each case considerably greater than that observed in classic late-onset FCD, even though the patients were decades younger at the time of surgery. This indicates a several-fold more rapid deposition of DM extracellular matrix, which is normally secreted throughout life by the corneal endothelium. In normal individuals, slow accretion of the normal DM membrane subjacent to the corneal endothelium has been characterized in considerable detail. This process has been likened to the growth of tree rings, in that the nature of this deposited matrix changes with time, leaving behind a complex laminar structure that provides a sequential record of the secretory activity of the endothelium. The most rapid growth and deposition of the first ~2 to 3 μm of DM takes place during fetal life, with the rate of growth slowing considerably after birth and infancy. The immunocytochemical and ultrastructural results in this study indicate that this entire growth period is abnormal and that pathology is not restricted to the most recently deposited layers of DM.
In this regard, our consistent finding that the most anterior layer of DM shows unusually high levels of collagen VIII indicates that the pathology associated with this mutation actually begins before birth. The irregular collagen VIII–rich material that extends vertically from the anterior edge of DM appears to represent sections of lamellar structures that were first laid down in early fetal life and that extended posteriorly during subsequent growth of DM. Horizontal discontinuities in the protein composition of DM, as observed dramatically in Figure 1C, and in the horizontal “lines” of collagen VIII reveal abrupt changes in the secretory function of the endothelial cells, which could be related to physiological changes in the individual or an episodic disease process. The posterior and latest layers of DM deposited in cases 2 and 3 are especially rich in not only collagen VIII, but also laminin, collagen IV, and fibronectin. These components appear in a complex and partially overlapping sequence that could reflect final changes in secretory function of stressed and dying endothelial cells.
Protruding shallow guttae were only observed in the least advanced L450W COL8A2 patient, suggesting that this asymmetric, localized deposition of DM begins early, but that it is somehow resurfaced by later growth of DM, producing a more uniform surface. Presumably the endothelial production of extracellular material coverage remains fairly complete until the final stages of decompensation, because we would otherwise expect clefts and abrupt changes in the thickness of DM, something that we do not observe. An interesting implication of this observation is that the ultimate dysfunction and death of the endothelium is not closely linked to the physical structure of the guttae themselves. It may instead be the result of increasing thickness and abnormal composition of the extracellular matrix that normally provides support to the endothelium. Although the tensile strength of the very dense collagenous components of DM is important for the integrity of the cornea, it is possible that as this layer exceeds a critical thickness, the transfer of solutes, nutrients, or growth factors from the corneal stroma is impeded, placing an ultimately lethal stress on the metabolically active endothelium. Whether the considerable compositional changes observed near the posterior boundary are a cause or result of this stress is an interesting question that cannot be answered at present.
In this study, the case 1 cornea has allowed us to look at the proteins and ultrastructure of the remaining endothelial cells. Although endothelial cells of normal cornea express similar amounts of the COL8A1 and COL8A2 isoforms, there is selective and greatly increased antibody signal to only COL8A2 within the endothelial cells of case 1 and case 2, whereas no cells are present in case 3. This increased density of COL8A2 may be related to altered properties of the mutant gene product and appears to explain the greater amounts of COL8A2 protein near the posterior edge of DM. Although this same region of DM also has elevated amounts of laminin, collagen IV, and fibronectin, the endothelial cells do not seem to contain significant amounts of these proteins. One possible reason for this difference might be that laminin, collagen IV, and fibronectin are rapidly secreted by the endothelial cells, whereas the COL8A2 is retained to a greater degree, possibly as a result of the L450W mutation in its highly conserved collagen helix domain,2 and may be disruptive in the formation of procollagen or its secretion. Confocal microscopy showed a granular, nonhomogenous distribution of COL8A2 in the cells. Immunoelectron microscopy further localized COL8A2 to the RER, where it appears primarily associated with the membrane-bound ribosomes, rather than inside the lumen of the RER. Whether this represents a pathological feature associated with this mutation remains to be determined.
Immunocytochemistry using antibodies to collagen 8A1 and 8A2, collagen 4, laminin, and fibronectin revealed a complex distribution that differed substantially from normal DM and endothelium. The most prominent observation was the high levels of collagen VIII in the endothelial cells and DM at various stages of the disease process. In the earlier stages of this disease, as in case 1, labeling with COL8A2 antibody revealed intensely stained vertically arranged collagen fibers traversing the anterior two thirds of DM. The COL8A1 staining was mostly colocalized with COL8A2 antibody labeling. Some of the remaining endothelial cells in case 1 and case 2 were intensely positive with COL8A2 antibody (Figure 3), which indicates these endothelial cells were highly active in producing more collagen type VIII protein. Hogan and colleagues7 reported that endothelial cells in patients with FCD had an increase of Golgi system and dilated endoplasmic reticulum, indicating an increase in protein synthesis of extracelluar fibrils and ground substance. In case 3 at the later stages of the disease process, COL8A2 staining showed an intensely labeled band in the posterior region of DM. In DM of case 2, collagen VIII staining showed features of both case 1 (collagen fiber-like structures in the DM) and case 3 (a band-like pattern in the posterior region of DM) and was heterogeneous in the immunolabeling pattern. The different collagen VIII distribution in DM as seen in these three cases may represent a remodeling process or may demonstrate various phenotypic subtypes in the early-onset FCD.
At the electron microscopy level, in FCD, DM is classically divided into four layers: an anterior banded layer (ABL), a posterior nonbanded layer (PNBL), a posterior banded layer, and a fibrillar layer.9–11 The ABL (about 3 μm) contains abundant collagen type VIII, which is formed in fetal life.12–15 The thickness of PNBL increases with age, averaging about 2 μm at age 10 years to 10 μm at age 80 years.12 In our cases, we have found aberrant accumulation of wide-space collagen in early-onset FCD with the COL8A2 mutation. The PNBL was intermixed with abundant aberrant banded collagen and spindle-shaped wide-space collagen, resulting in the absence of the PNBL, which was also reported in one of the 11 cases of FCD in Bourne and associates’ study.9 In that study, this patient was one of the youngest and could be an example of early-onset FCD. In case 1, excessive disorganized banded structures of wide-space collagen were present in much of the posterior region of DM, just anterior to the degenerated endothelial cells. Similar structures are present in FCD, where wide-space collagen is seen in the posterior collagenous layer (PCL).11 This observation indicates that it is possible that FCD also involves some degree of abnormal collagen VIII synthesis or assembly. However, as collagen VIII is one of the major structural components16 of DM, it is not surprising to see the same scattered wide-space collagen structures in the PCL of patients with aphakic bullous keratopathy,11,17 where endothelial cell function is severely impaired and DM is increased in thickness, but to a lesser extent than FCD.
Wide-space collagen fibers were first described in the eye, and Hogan and colleagues7 proposed that FCD pathology may be the result of spatial disorganization of an otherwise normal DM, because individual fibrils had normal dimensions and structure. In corneal endothelial cells, our immunoelectron microscopy demonstrated COL8A2 labeling of the RER membrane. The endothelial cell in case 1 displayed an abundant, unusual RER, with features previously described in type I cells by Iwamoto and DeVoe.10 This unusual RER was also reported in FCD by Hogan and colleagues in 1974.7 The intense labeling of gold particles in the RER may indicate that endothelial cells are active in COL8A2 protein synthesis and possibly are responsible for an accumulation of mutant COL8A2 protein.
In normal DM, different subunits of collagen IV are located in the stromal face or posterior nonbanded region of the DM. Fibronectin was observed in the stromal face, whereas laminin was found in the endothelial face or subendothelial region of the DM.18,19 In case 1, we found intense labeling of laminin and fibronectin in the posterior surface of the DM, and collagen IV showed more diffuse staining in the full thickness of DM. In this corneal tissue, many shallow guttae were present in the posterior surface of the DM, which stained markedly positive with the laminin antibody. In case 3, with more advanced disease, collagen IV and fibronectin staining displayed a thick stratum in the posterior-most aspect of DM. In some regions of DM, a loose fibrillar layer covered the buried guttae in DM. With the double fluorescent antibody labeling technique, we demonstrated that this fibrillar layer was abundant with collagen IV and fibronectin. The fibrillar layer was reported in the previous studies in late-onset FCD and bullous keratopathy.9–11,17 Bourne and colleagues9 proposed that the fibrillar layer was thicker when there was severe corneal edema, and the formation of this added layer could be a secondary response to severe endothelial cell damage late in the disease process. Laminin in case 3 was located anterior to collagen IV and fibronectin, and was intermixed with COL8A2 staining in some areas. In areas with guttae, laminin staining was intense along the profile of the posterior surface of the nodules on the posterior aspect of DM. Similar elevated levels of COL4 and laminin have also been found in DM of the iridocorneal endothelial syndrome and in congenital hereditary endothelial dystrophy.20,21
In this series, the first patient has shallow guttae and many remaining endothelial cells, the second has few endothelial cells and no guttae, and the third has no remaining endothelial cells. If it is assumed that these patients represent different time points in the disease process, shallow guttae appear first as the result of irregular matrix deposition by the endothelium. Subsequent to this, the endothelium secretes a qualitatively different extracellular matrix, one that is rich in laminin, collagen IV, and fibronectin. This final secretion of the stressed endothelial cells buries the guttae under a more uniform and flattened layer.
PEER DISCUSSION
DR MARK J. MANNIS
In 1890, Arthur Groenouw described two corneal dystrophies which he felt were variants of the same disease—Groenouw dystrophies Type I and II. Over the next fifty years, the significant differences in clinical appearance and observed patterns of heritability led to the conclusion that what we know as macular and granular dystrophies were two distinctly different disorders with very different clinical manifestations. Indeed, for most of the next century, the way in which we described dystrophic disorders was based on phenotype. In the 1990s, however—a century after Groenouw’s report—two important events led to a re-evaluation of the purely phenotypic classification of the corneal dystrophies. One was the observation of clinical entities such as Avellino dystrophy –which represented a clinical admixture of granular and lattice dystrophies. And the other was the development of advanced tools of molecular genetic analysis. The confluence of these two events rendered conditions ripe to look at the clinical dystrophies from the viewpoint of molecular genetics rather than pure clinical phenotype. Techniques of molecular genetics, immunohistochemistry, and high resolution microscopy have engendered the need to re-define our concept of the corneal dystrophies and their classification—and, perhaps, ultimately even their management. Well over 10 chromosome loci have now been identified and linked to corneal dystrophies. An example is the Beta-ig-h3 gene that encodes for the protein keratoepithelin and its relationship to several phenotypically distinct stromal dystrophies.
We now know that several entities that we once considered distinct disorders (granular, lattice, Avellino, and Reis-Bucklers’ dystrophy) are all linked to chromosome 5q31 and may actually be allelic forms in which keratoepithelin proteins are abnormally or excessively produced. Other disorders that have been linked to specific gene loci include Meesman epithelial dystrophy, Thiel-Behnke dystrophy, gelatinous drop-like dystrophy, and congenital hereditary endothelial dystrophy among others.
In this fascinating report by Gottsch and colleagues, building on their previous work, the authors have employed immunohistochemistry and electron microscopy to characterize further a rare and clinically distinct early onset, familial form of Fuchs corneal dystrophy caused by mutation in the COL8A2 gene.4 They have effectively demonstrated that the pathologic changes in Descemet’s membrane of the COL8A2 L450W mutants include increased growth of extracellular matrix, attenuation and loss of the endothelium, and abnormal deposition of collagen Type VIII, laminin, and fibronectin. In previous work, using gene mapping and mutation analysis, the genes responsible for early and late onset Fuchs corneal dystrophy have been demonstrated to be different. The present study adds detailed immunopathological characterization and patient-to-patient variations in this disorder to understand better the underlying pathology and its variability at different stages of development.
The dominant, fully penetrant L450W COL8A2 variant of Fuchs dystrophy appears in early childhood and leads to endothelial dysfunction in the first two decades, in contrast to the more common form of Fuchs dystrophy with which we are all well acquainted. This study demonstrates that, not only are the clinical patterns and findings distinct, but that the microscopy in these patients show that it is structurally and developmentally different, as described in detail by Dr. Gottsch. Moreover, the authors have convincingly suggested that the differing distributions of Collagen VIII respectively in the three reported cases may be the histopathologic counterparts of various phenotypic subtypes of the disorder.
This study correlates immunohistopathology, specific clinical findings, and electron microscopic ultrastructure with a clearly identified gene-linked disorder. As such it bridges the phenotypic and genetic approaches to the definition of the dystrophies.
Two important initiatives currently underway should be mentioned with regard to the definition and classification of the corneal dystrophies—one general and one related specific ally to Fuchs dystrophy. The need to classify the dystrophies more logically and accurately has emanated directly from the revelations of molecular genetics. Accordingly the Cornea Society has sponsored the formation on the International Committee on Classification of Corneal Dystrophies (IC3D) Committee. This committee is comprised of an international group of clinicians, geneticists and pathologists under the direction of Dr. Jayne Weiss, and its goal is to devise a new nomenclature for the corneal dystrophies which is embraced globally by clinicians, geneticists and pathologists. With rapid advances in genotyping, the nomenclature of the corneal dystrophies has become archaic. Corneal dystrophies with different names and phenotypes may actually have similar genotypes. Conversely, corneal dystrophies categorized together with similar phenotypes may be distinct genotypically. The nomenclature is no longer adequate to reflect our current level of understanding in this field. It is time to develop a more meaningful nomenclature that can easily be used by clinicians and that more accurately reflects not only the phenotype noted on clinical exam but also the associated genetic defect. The committee is now in the process of "weeding out" the misinformation to create an up-to-date accurate information base. At the same time, each dystrophy is being categorized into a new and informative classification system to be finalized at the 2006 AAO and prepared for publication.
The second of these initiatives is the NIH-sponsored prospective multicenter trial—the Fuchs Endothelial Corneal Dystrophy Study—designed to elucidate the genetic underpinnings of Fuchs corneal dystrophy. Based at Case Western Reserve and under the direction of Jonathan Lass, the FECD has three aims: 1) to identify cases with advanced Fuchs dystrophy and characterize family clustering 2) to conduct a genome-wide scan utilizing DNA from the index cases and families (Est. N = 500 families, > 500 sib pairs) Model free linkage analysis will then be conducted using DNA markers and clinical data 3) and finally, the study will identify candidate genes using investigations of a limited number of families such as those with the COL8A2 mutation and will investigate the importance of these genes on a more global basis by characterizing their role in the larger sample Hopefully the FECD study will lead to novel insights into the etiology of Fuchs dystrophy and the biology of the corneal endothelium.
I congratulate the authors on a meticulously performed study that will add to our understanding not only of this rare dystrophic corneal disorder but to the way in which we implement current technology to unravel the secrets of the dystrophies. Our ability to manage these patients in the future will hinge both on accurate phenotypic diagnosis as well as genetic analysis that may lead to novel therapeutic interventions.
REFERENCES
- 1.Groenouw A. Knötchenförmige Hornhauttrübungen (noduli corneae) Arch Augenheilkunde. 1890;21:281–289. [Google Scholar]
- 2.Holland EJ, Daya SM, Stone EM, et al. Avellino corneal dystrophy. Clinical manifestations and natural history. Ophthalmology. 1992;99:1564–1568. doi: 10.1016/s0161-6420(92)31766-x. [DOI] [PubMed] [Google Scholar]
- 3.Bron AJ. Genetics of the corneal dystrophies: what we have learned in the past twenty-five years. Cornea. 2000;19:699–711. doi: 10.1097/00003226-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Gottsch JD, Zhang C, Sundin OH, et al. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–4511. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
DR JAMES MCCULLEY
Is what you’re describing as your early-onset Fuchs, what we used to call clinically, and I guess more appropriately phenotypically, congenital hereditary corneal endothelial dystrophy, or are those two yet different?
DR JAY KRACHMER
Roughly 30 years ago, I went to Washington to look at Mal McGovern’s patients and I was just looking with the slit lamp. Now we have sophisticated techniques with histopathology examinations and molecular genetics examinations. We should all appreciate the present efforts of the Cornea Society in attempting to categorize the corneal dystrophies. Years ago I traveled to Washington from Iowa City to see some of these patients and they looked different from what we normally think of as Fuchs Corneal Dystrophy. With the slit lamp, I did not see the guttata and indeed they are mainly buried. I was struck by the young age of the patients, although we have all seen classic Fuchs Dystrophy among families where the youngest can be in their twenties and may even require corneal transplants in their twenties. These patients look really different and maybe they will not be classified as Fuchs Dystrophy but rather have a different name. Maybe we will find enough genetic and histopathologic characteristics in common that they will combine them into one group.
DR RICHARD C. TROUTMAN
Have you had any experience to report on the slit lamp examinations of those patients who have had corneal grafts?
DR JOHN D. GOTTSCH
Dr. McCulley brings up a good point in that congenital hereditary endothelial dystrophy can look similar in the clinical picture of advanced stages of patients with the L450W COL8A2 mutation. Histopathologically however, congenital hereditary endothelial dystrophy looks very different than the L450W COL8A2 Fuchs dystrophy corneas. In those that we have examined, the massive COL8A2 staining noted in the early onset Fuchs disease is missing. In congenital hereditary dystrophy the disease is noted early after birth. Patients with the L450W mutation do have a problem early in the first decade of life with scattered guttae, but by in large the corneas are without stromal edema and the vision is not affected.
As for Dr. Krachmer’s comments, we too were pleased that we are able to unravel this mystery about these different types of Fuchs Dystrophy. This clinical type of Fuchs dystrophy could be referred to simply by its mutation: the L450W COL8A2 We are looking to the future for some type of gene therapy to help the afflicted patients in this family.
Dr Troutman asks if we have any experience with slit lamp examination of patients who have corneal grafts for the L450W COL8A2 mutation. Yes, I have performed transplants on three patients and all have done well without any unusual problems or recurrence of the dystrophy. I have about a two year of follow up for two of the patients.
REFERENCES
- 1.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 2.Gottsch JD, Sundin OH, Liu SH, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46:1934–1939. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 3.Sundin OH, Jun AS, Broman KW, et al. Linkage of late-onset Fuchs corneal dystrophy to a novel locus at 13pTel-13q12.13. Invest Ophthalmol Vis Sci. 2006;47:140–145. doi: 10.1167/iovs.05-0578. [DOI] [PubMed] [Google Scholar]
- 4.Sundin OH, Broman KW, Chang HH, et al. A common locus for late-onset Fuchs corneal dystrophy maps to 18q21.2–q21.32. Invest Ophthalmol Vis Sci. 2006;47:3919–3926. doi: 10.1167/iovs.05-1619. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch JD, Zhang C, Sundin OH, et al. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–4511. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
- 6.Magovern M, Beauchamp GR, McTigue JW, et al. Inheritance of Fuchs’ combined dystrophy. Ophthalmology. 1979;86:1897–1923. doi: 10.1016/s0161-6420(79)35340-4. [DOI] [PubMed] [Google Scholar]
- 7.Hogan MJ, Wood I, Fine M. Fuchs’ endothelial dystrophy of the cornea. 29th Sanford Gifford Memorial lecture. Am J Ophthalmol. 1974;78:363–383. doi: 10.1016/0002-9394(74)90224-4. [DOI] [PubMed] [Google Scholar]
- 8.Waring GO, 3rd, Rodrigues MM, Laibson PR. Corneal dystrophies. II. Endothelial dystrophies. Surv Ophthalmol. 1978;23:147–168. doi: 10.1016/0039-6257(78)90151-0. [DOI] [PubMed] [Google Scholar]
- 9.Bourne WM, Johnson DH, Campbell RJ. The ultrastructure of Descemet’s membrane. III. Fuchs’ dystrophy. Arch Ophthalmol. 1982;100:1952–1955. doi: 10.1001/archopht.1982.01030040932013. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto T, DeVoe AG. Electron microscopic studies on Fuchs’ combined dystrophy. I. Posterior portion of the cornea. Invest Ophthalmol. 1971;10:9–28. [PubMed] [Google Scholar]
- 11.Yuen HK, Rassier CE, Jardeleza MS, et al. A morphologic study of Fuchs dystrophy and bullous keratopathy. Cornea. 2005;24:319–327. doi: 10.1097/01.ico.0000148288.53323.b2. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet’s membrane. I. Changes with age in normal corneas. Arch Ophthalmol. 1982;100:1942–1947. doi: 10.1001/archopht.1982.01030040922011. [DOI] [PubMed] [Google Scholar]
- 13.Levy SG, Moss J, Sawada H, et al. The composition of wide-spaced collagen in normal and diseased Descemet’s membrane. Curr Eye Res. 1996;15:45–52. doi: 10.3109/02713689609017610. [DOI] [PubMed] [Google Scholar]
- 14.Sawada H, Konomi H, Hirosawa K. Characterization of the collagen in the hexagonal lattice of Descemet’s membrane: its relation to type VIII collagen. J Cell Biol. 1990;110:219–227. doi: 10.1083/jcb.110.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura Y, Konomi H, Sawada H, et al. Tissue distribution of type VIII collagen in human adult and fetal eyes. Invest Ophthalmol Vis Sci. 1991;32:2636–2644. [PubMed] [Google Scholar]
- 16.Hopfer U, Fukai N, Hopfer H, et al. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005;19:1232–1244. doi: 10.1096/fj.04-3019com. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet’s membrane. II. Aphakic bullous keratopathy. Arch Ophthalmol. 1982;100:1948–1951. doi: 10.1001/archopht.1982.01030040928012. [DOI] [PubMed] [Google Scholar]
- 18.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 19.Marshall GE, Konstas AG, Lee WR. Immunogold fine structural localization of extracellular matrix components in aged human cornea. I. Types I–IV collagen and laminin. Graefes Arch Clin Exp Ophthalmol. 1991;229:157–163. doi: 10.1007/BF00170550. [DOI] [PubMed] [Google Scholar]
- 20.Levy SG, McCartney AC, Sawada H, et al. Descemet’s membrane in the iridocorneal-endothelial syndrome: morphology and composition. Exp Eye Res. 1995;61:323–333. doi: 10.1016/s0014-4835(05)80127-7. [DOI] [PubMed] [Google Scholar]
- 21.Sekundo W, Marshall GE, Lee WR, et al. Immuno-electron labelling of matrix components in congenital hereditary endothelial dystrophy. Graefes Arch Clin Exp Ophthalmol. 1994;232:337–346. doi: 10.1007/BF00175985. [DOI] [PubMed] [Google Scholar]