Figure 2. caPKCε overexpression increases MARCKS phosphorylation in NRVM and ARVM.
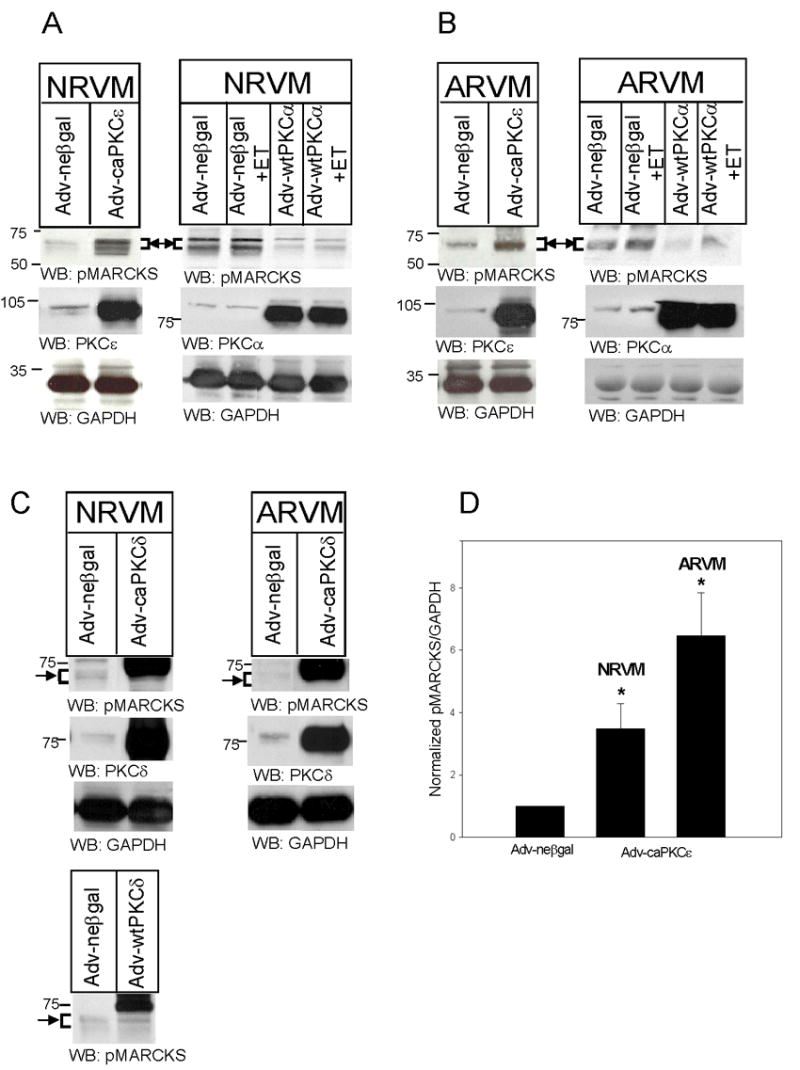
NRVM (A) and ARVM (B) were infected with Adv-neβgal and Adv-caPKCε, or Adv-neβgal and Adv-wtPKCα (25MOI, 24h), and then treated with ET (10nM, 10 min). In Panel C, NRVM and ARVM were infected with Adv-neβgal, Adv-caPKCδ, or Adv-wtPKCδ (25MOI, 24h). Western blots (50μg of extracted protein) were probed with either an antibody specific for MARCKS phosphorylated at Serine 160/163, antibodies specific for PKC-ε, -δ, or -α, or an antibody that recognizes GAPDH. Western blots depicted in Panel C were run for a longer period of time in order to maximize the separation of bands. The positions of molecular weight standards are indicated to the left of each blot. Arrow and bracket sets indicate band area of pMARCKS. Panel D depicts the quantitative analysis of 4–5 Western blotting experiments. Levels of phosphorylated MARCKS were first normalized to their respective GAPDH, and then normalized to levels in neβgal-expressing cells. Data are means ± SEM; P<0.05 vs. Adv-neβgal.
