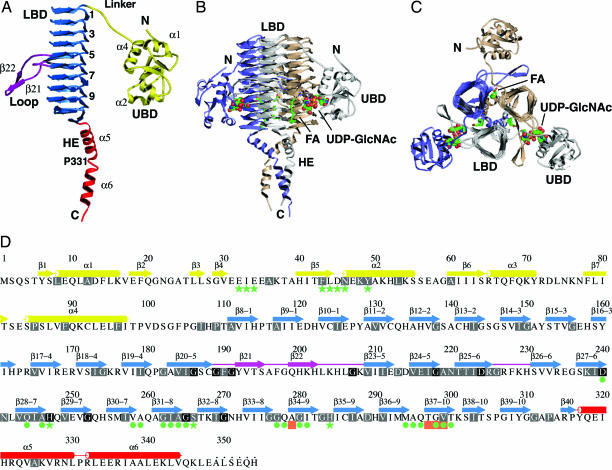
Fig. 2.
Structure of LpxD. (A) Ribbon diagram of a subunit. The UBD is yellow, the LBD is blue, loops are magenta, and the HE is red. Selected elements of secondary structure are labeled, and the coils are numbered. (B) The trimer. The view is parallel to the noncrystallographic symmetry threefold axis. Subunits are colored gray, wheat, and slate, and the domains of the gray subunit are labeled. UDP-GlcNAc (complex II) is represented by spheres, and palmitic acid is represented by sticks. The atoms of the ligands are colored as follows: C, green; N, blue; O, red; P, yellow. (C) Orthogonal view of the trimer. (D) Primary and secondary structure. β-strands are depicted by arrows, and α-helices are depicted by cylinders. Colors are as described in A. Disordered residues at the C terminus are marked by dots. Highly conserved residues (>60% identity in 85 sequences) are highlighted in gray, and strictly conserved residues (100% identity) are in black. Green stars and circles indicate residues that interact with UDP-GlcNAc and palmitic acid, respectively. Salmon boxes represent sites of conditionally lethal point mutations in E. coli and S. typhimurium LpxD.