Figure 2. Phospho-L13a Blocks Assembly of the 48S Complex.
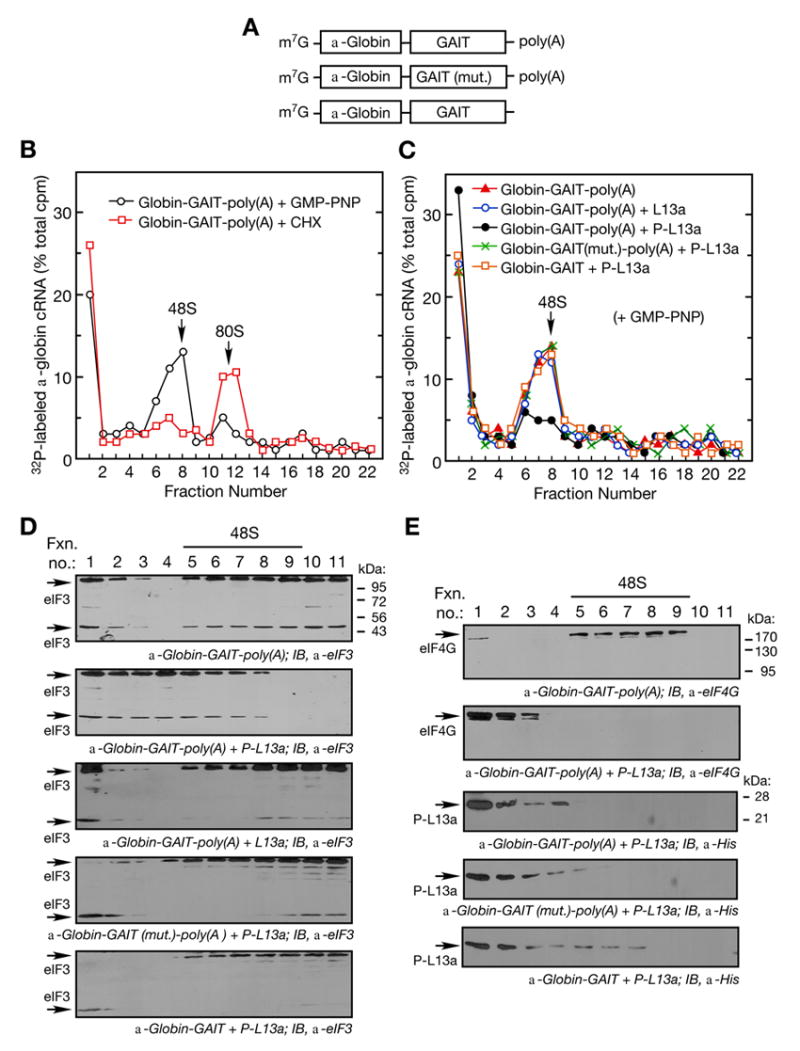
(A) Schematic of α-globin RNA constructs used in translation initiation reactions. Shown are α-globin RNA reporters carrying the wild-type (top) or mutant (middle) GAIT element with a poly(A) tail, and α-globin RNA carrying the wild-type GAIT element without a poly(A) tail (bottom).
(B) Ribosomal complex formation on GAIT element-containing RNA. Translation initiation reactions containing 32P-labeled, α-globin-GAIT-poly(A) reporters (300,000 cpm) were reconstituted in RRL in presence of GMP-PNP (black ○, 2 mM) or cycloheximide (red □, CHX, 0.5 mM) and resolved by sucrose density gradient centrifugation. Radioactivity in fractions was determined and peak positions of 48S and 80S complexes are indicated.
(C) Phospho-L13a blocks 48S complex formation on a GAIT element-containing RNA in a poly(A)-dependent way. Translation initiation reactions were reconstituted in a RRL with GMP-PNP (2 mM) as in Fig. 2B, but in the presence of His-tagged, phospho-L13a (5 μg). Conditions tested were: α-globin-GAIT-poly(A) (red ▲), α-globin-GAIT-poly(A) + L13a (blue ○), α-globin-GAIT-poly(A) + phospho-L13a (black ●), α-globin-GAIT (mut.)-poly(A) + phospho-L13a (green ×), α-globin-GAIT (no poly(A) tail) + phospho-L13a (orange □). The reaction was subjected to sucrose density gradient centrifugation, fractions (0.75 ml) were collected, and radioactivity determined.
(D) Phospho-L13a prevents formation of eIF3-containing 48S complex. Gradient fractions from experiments with α-globin-GAIT-poly(A) reporter and buffer control (top), phospho-L13a (middle), and unmodified L13a (bottom) were subjected to TCA precipitation followed by IB analysis with anti-eIF3 antibody. Two eIF3 subunits (44 and 110 kDa) are indicated.
(E) Migration of eIF4G and phospho-L13a in the sucrose gradient. Gradient fractions from Fig. 2C containing α-globin-GAIT-poly(A) reporter and buffer control (top panel) or phospho-L13a (2nd panel) were subjected to TCA-precipitation followed by IB analysis with anti-eIF4G antibody. Migration of phospho-L13a in the presence of α-globin-GAIT-poly(A) (3rd panel), α-globin-GAIT (mut.)-poly(A) (4th panel), or α-globin-GAIT (bottom panel) was determined by IB analysis with anti-His antibody.
