Fig. 7.
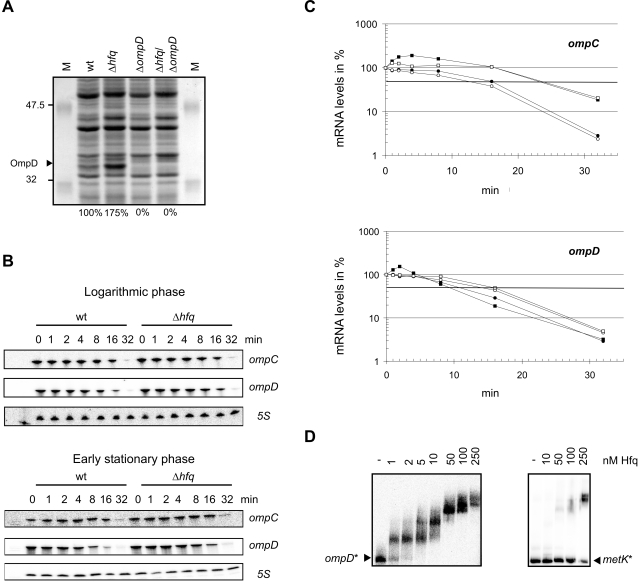
Hfq is essential for growth rate-dependent repression of OmpD. A. SDS-PAGE analysis of total protein prepared from wild-type, Δhfq, ΔompD and Δhfq/ΔompD bacteria grown to early stationary phase. OmpD protein levels as quantified by fluorescent staining (not shown) are given below each lane. B. Northern blot detection of ompC and ompD mRNA levels of wild-type and Δhfq bacteria grown to either logarithmic or early stationary phase prior to (0 min) and within 32 min of rifampicin treatment. 5S sRNA probing (loading control) is shown below each panel. C. Decay of ompC and ompD mRNA upon rifampicin treatment as derived from quantification of the Northern blot signals shown in (B). Logarithmic phase, wild-type (filled circles) or Δhfq (open circles); early stationary phase, wild-type (filled squares) or Δhfq (open squares). D. Hfq binds to ompD 5′ UTR RNA in vitro (gel mobility shift assay). Left panel: 1 nM of 32P-labelled ompD was incubated with increasing concentrations of Hfq protein (given above the lanes). Following a 15 min incubation at 37°C samples were run on a native 6% gel. Shown is an autoradiograph of the gel. A control gel shift assay with an Hfq-independent RNA derived from the metK 5′ UTR is shown in the right panel.