Abstract
Neuropathic pain refers to pain that originates from pathology of the nervous system. Diabetes, infection (herpes zoster),nerve compression, nerve trauma, “channelopathies,” and autoimmune disease are examples of diseases that maycause neuropathic pain. The development ofbothanimal models and newer pharmacological strategies has led to an explosion of interest in the underlying mechanisms. Neuropathic pain reflects both peripheral and central sensitization mechanisms. Abnormal signals arise not only from injured axons but also from the intact nociceptors that share the innervation territory of the injured nerve. This review focuses on how both human studies and animal models are helping to elucidate the mechanisms underlying these surprisingly common disorders. The rapid gain in knowledge about abnormal signaling promises breakthroughs in the treatment of these often debilitating disorders.
Chronic pain has been estimated to affect one-sixth of the population. The phrase “neuropathic pain” came into common use only in the last decade and increasingly has been appreciated as a frequent source of chronic pain, perhaps trailing only osteoarthritis as a cause. Neuropathic pain results from pathology in the nervous system. Consider the following clinical presentation:
A 47-year-old woman presented for consultation for complaints of pain on the right chest wall. The patient underwent a mastectomy on the right side as a treatment for cancer 5 years previously. As the surgical pain faded, the patient noted increasing chest-wall pain that extended well beyond the surgical borders. Clothing lightly touching the skin increased the pain. Reconstructive surgery was deferred because of concerns about the ongoing pain. In addition to the ongoing burning pain, the patient also complained of sudden “pain attacks” one to several times a day. These attacks lasted seconds to minutes and were described as debilitating. Examination revealed a well-healed surgical scar. Light stroking of the skin provoked significant pain in an area from the clavicle down to the T8 dermatome. Despite the pain to light tactile stimuli, the patient also had areas of decreased sensibility as demonstrated by the inability to detect a fine probe applied to the skin.
The above case represents a classical presentation of a patient with neuropathic pain. The notable features that point to neuropathic processes are as follows:
Widespread pain not otherwise explainable
Evidence of sensory deficit
Burning pain
Pain to light stroking of the skin
Attacks of pain without seeming provocation
The liability for pain appears to vary from person to person, from nerve to nerve, between males and females, and even with age. What appears to be the same lesion may induce no pain in one person but severe pain in another. In addition to ongoing pain (i.e., stimulus-independent pain), patients may have heightened pain to stimuli applied to their skin. This enhanced stimulus-dependent pain is called hyperalgesia. In some patients, lightly stoking the skin may evoke pain. This pain to light touch is often called allodynia (Treede et al., 1992).
In this review, we will focus on the mechanisms of neuropathic pain. Lesions of the CNS (e.g., spinal cord) and peripheral nerves may lead to pain, but the majority of experimental studies have focused on consequences of lesions of peripheral nerves, and therefore, we will focus on this area in this review.
Anatomical Considerations
Neuropathic pain is distinguished from other pain conditions where the pain generator begins with disease of nonneural tissues. These nonneuropathic pain entities are said to be nociceptive and include conditions such as osteoarthitis and inflammatory pain. By definition, neuropathic pain originates from a lesion of the nervous system. Innumerable diseases may be the culprits. Examples include autoimmune disease (e.g., multiple sclerosis), metabolic diseases (e.g., diabetic neuropathy), infection (e.g., shingles and the sequel, postherpetic neuralgia), vascular disease (stroke), trauma, and cancer. A rule without apparent exception is that the lesion leading to pain must directly involve the nociceptive pathways (Boivie et al., 1989). Accordingly, for example, lesions of the medial lemniscal system (e.g., dorsal columns) do not induce pain (Cook and Browder, 1965).
Whereas evidence supports the hypothesis that pain-generating lesions of the nervous system must involve the nociceptive pathways, the converse clearly does not hold up. Namely, not all lesions of nociceptive pathways induce pain. A lesion of the peripheral nerve may induce pain, but simply severing dorsal roots seems to have little chance of creating lasting pain (Li et al., 2000). For example, dorsal roots are cut to treat spasticity and sometimes to remove tumors. The authors know of no case of neuropathic pain arising from these types of lesions in man. Here, we do not take up the issue of CNS lesions, but it is nevertheless worth noting that though spinal cord lesions carry a substantial risk of inducing pain, some evidence suggests that the lesion must include the gray matter. Vireck has found that simple cordotomy where only the white matter is lesioned does not induce abnormal pain behavior in a primate model, whereas lesions that include the gray matter may (Vierck and Light, 1999). Lesions of the brainstem and thalamus carry a risk of causing pain if the nociceptive pathways are involved (Boivie, 2006). In nearly all of these cases, there is the paradoxical juxtaposition of ongoing pain and a sensory deficit to noxious stimulation (even when hyperalgesia is present). Lesions confined to the cortex appear not to be associated with abnormal pain (but it is also unclear whether lesions confined to the cerebral cortex induce deficits of pain sensibility [Head and Holmes, 1911]).
Nervous System Diseases Associated with Pain
As noted above, a rich variety of diseases of the nervous system are associated with pain (Table 1) (Scadding and Koltzenburg, 2006). In the introduction, a case of traumatic painful neuropathy was presented (Campbell, 2001). The offending lesion may involve a simple axotomy (cutting of the nerve) or merely nerve entrapment. Ongoing pain may be the only manifestation, but in many cases, hyperalgesia with pain to light-stroking stimuli (allodynia) may be manifest. In some cases, there is striking cooling hyperalgesia, where mild lowering of skin temperature exacerbates the pain. Weir Mitchell described a striking variant of neuropathic pain that he termed causalgia (Lau and Chung, 2004). He noted this condition in patients who had missile lesions of an extremity. Patients presented with striking edema, profound autonomic features (abnormal sweating and either hot or cold skin), and devastating pain. A similar presentation can occur without a clear nerve injury, and presently, this is referred to as “complex regional pain syndrome (CRPS), type 1.” Where a clear nerve lesion is manifest, the condition is referred to as CRPS, type 2 (Stanton-Hicks et al., 1995).
Table 1.
Examples of Neuropathic Pain Conditions
| Disease | Pathology | Symptoms | Hyperalgesia | Special Features | ||
|---|---|---|---|---|---|---|
| Cooling | Heat | Mechanical | ||||
| Traumatic neuropathy | Axotomy distal to DRG | Ongoing pain | ++ | + | ++ | Great variety of presentations |
| Tic douloureux | Compression (often vascular) of trigeminal nerve near brainstem | Lightening-like attacks of pain | ? | ? | +++ | Mechanical stimuli evoke attacks of pain |
| Painful diabetic neuropathy | Length-dependent neuropathy | Burning pain in the feet | − | + | +/− | Neuropathy sometimes affects small fibers exclusively |
| Postherpetic neuralgia | Results from damage by herpes zoster infection of peripheral nerve | Burning pain | +/− | +/− | ++ | Pain follows the distribution of the affected dermatomes |
One of the classic examples of neuropathic pain is Tic douloureux. Without treatment, this is a debilitating disorder that involves attacks of severe pain in the facial area (also referred to as trigeminal neuralgia). Often there is little or no pain between attacks. The lightening-like attacks are referred to one of the dermatomes (V1, V2, or V3). Light touching of the skin in a so-called trigger zone suffices to evoke an attack. The disease appears to be associated with mechanical distortion at the entry zone of the nerve root to the brainstem. Demyelination may be seen at the compression site. Nerve compression from an aberrant blood vessel is one of the more common causes (Elias and Burchiel, 2002).
Another classical neuropathic pain condition is painful diabetic neuropathy (Dyck et al., 2000). Diabetes often causes a length-dependent neuropathy (meaning that the longest axons in the peripheral nerve are most vulnerable). Patients report bilateral burning pain in the toes and feet. Quantitative sensory testing reveals decreased pain sensibility (with or without decreased touch sensibility).
Postherpetic neuralgia is a complication of shingles and is an example of how an infection can lead to pain. Shingles results from an activation of the herpes zoster virus that takes up residence in the dorsal root ganglion after a chickenpox infection. The shingles eruption consists of blisters that follow the dermatome(s) of one or more spinal nerves. The blisters heal in time, but the pain may continue. Allodynia is a particularly prominent feature of postherpetic neuralgia. This allodynia may be present even with loss of C-fiber innervation of the epidermis.
The clinical manifestations vary with the type of disease (Table 1). These variations suggest different mechanisms. These differences in mechanism may also be reflected in responses to therapy. Tic douloureux responds nicely to treatment with the anticonvulsant carbamazepine. Responses to carbamazepine for other conditions are typically disappointing. Painful diabetic neuropathy leads to ongoing pain, but allodynia is distinctly unusual, and cooling may relieve pain. In contrast, allodynia is prominent in traumatic neuropathy and cooling often causes severe pain.
Animal Models of Neuropathic Pain
Whereas the distinction between nociceptive and neuropathic pain has utility, in the actual clinical setting, the mechanisms are often intertwined (e.g., back pain with radiculopathy). Fortunately, however, this need not be the case with regard to animal models. A great advance in the study of neuropathic pain emanated from the discovery that placement of loose chromic ligatures on the sciatic nerve in rat brought about behavior that appeared analogous to human neuropathic pain conditions (Bennett and Xie, 1988). The rats engaged in protective behavior and had lowered thresholds to heat, cooling, and mechanical stimuli.
Subsequent work indicated that an idiosyncratic immune-mediated response to the chromic suture played a major role in the development of the model (Maves et al., 1993). The nerve swelled, leading to nerve compression and axotomy. But can an axotomy by itself induce pain? The answer is unequivocally yes. Chung and colleagues (Kim and Chung, 1992) devised a now frequently used model in rat whereby one or more spinal nerves that innervates the foot is cut (SNL model in Figure 1A). A substantial innervation of the foot remains, reflecting input from the adjacent spinal nerves. This remaining innervation allows tests for hyperalgesia to be undertaken. A simple axotomy provides a foundation by which to study mechanism in diseases where axotomy is part of the disease process. Some of the common traumatic nerve injury models are illustrated in Figure 1A. These injuries lead to hyperalgesia (Figures 1B and 1C).
Figure 1. Animal Models of Neuropathic Pain.
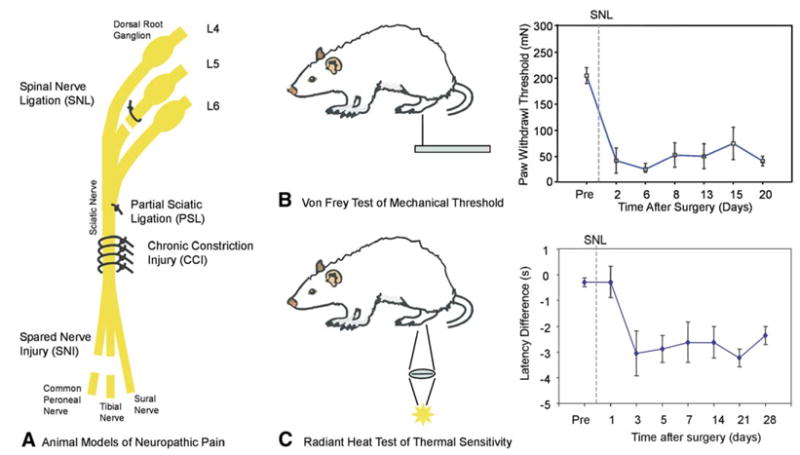
(A) Four different nerve injury models are shown. In the spinal nerve ligation (SNL) model, one or more spinal nerves going to the foot are ligated and cut (Kim and Chung, 1992). In the partial sciatic ligation (PSL) model, a portion of the sciatic nerve is tightly ligated (Seltzer et al., 1990). The chronic constriction injury (CCI) model involves placement of four loose chromic-gut ligatures on the sciatic nerve. An immune response to the sutures leads to nerve swelling and nerve constriction. In the spared nerve injury (SNI) model, the common peroneal and tibial nerves are cut, sparing the sural nerve (Decosterd and Woolf, 2000). In each model, only a portion of the afferents going to the foot are lesioned.
(B and C) Each of these nerve injury models leads to hyperalgesia, which is manifest by enhanced responses to mechanical, heat, and/or cooling stimuli. (B) To test for mechanical hyperalgesia, Von Frey monofilaments with different bending forces are applied to the plantar surface of the foot. The threshold force for paw withdrawal decreases dramatically after the nerve injury (adapted with permission [Li et al., 2000])
(C) To test for heat hyperalgesia, a radiant heat source is focused onto the plantar surface of the foot, and the reaction time for paw withdrawal is measured. The difference in reaction time between the ipsilateral and contralateral foot is calculated. After the SNL, the withdrawal of the ipsilateral foot is faster than the contralateral foot (negative latency difference), indicating the presence of heat hyperalgesia (adapted from Kim and Chung [1992] reprinted from Pain, pp. 355–363, copyright 1992, with permission from the International Association for the Study of Pain).
Data are presented as mean ± SEM.
Trauma has not been the only model used in studies of neuropathic pain. One of the leading causes of pain in humans is diabetic neuropathy. Injection of streptozotocin leads to an animal model of diabetes and is associated with the development of neuropathy, similar to what is seen in humans. Hyperalgesia can be measured in rodent models, thus providing a means to study treatments and mechanisms of pain in this neuropathy model (Ahlgren and Levine, 1993). One of the dose-limiting problems in chemotherapy is neuropathy, and these neuropathies are often associated with pain. Animal models of paclitaxel and vincristine-induced painful neuropathies have been developed (Flatters and Bennett, 2004).
Though hyperalgesia is relatively easy to demonstrate in animal models, the measurement of ongoing pain is more problematic. This issue is important because ongoing pain without clear hyperalgesia appears to be a common occurrence in humans (Basbaum et al., 2006). Substantial denervation of a limb in rat often leads to autotomy behavior. Some have advocated that autotomy is an indication of ongoing pain, the presumed rationale being that the animal desires to eliminate the painful body part (Devor, 1991). An alternative explanation is that the animal chews an anesthetic part for reasons unrelated to pain. Some have argued that spontaneous foot lifting may provide a behavioral measure of spontaneous pain (Djouhri et al., 2006). Alternative measures of ongoing pain involve the use of cellular markers of increased neuronal activity. Increased expression of the immediate early gene protein, c-Fos, in the dorsal horn (and perhaps other more rostral sites) is an example (Bullitt, 1990). Small-animal functional magnetic resonance imaging (fMRI) and/or PET imaging may in the future offer alternative measures.
Animal models of neuropathic pain in some cases have been inconsistent with human models. Allodynia in painful diabetic neuropathy in humans is infrequent yet appears to be robust in rat models. NK1 antagonists appeared to have promise for treatments of pain based on animal models yet to date have not proven useful in patients. Vierck (2006) argues that “reflex” measures of pain in animal neuropathic models are intrinsically flawed and are neither sensitive nor specific predictors of drug efficacy in man. For example, he points out that the paw-withdrawal threshold method tests motor neuron response rather than simply providing a measure of pain. Moreover, he indicates that rostral signaling pathways may be ignored when one merely measures the paw-withdrawal threshold. His solution is to utilize operant models.
However, operant measures may be flawed as well in that they introduce other complexities such as motivational factors that may be extraneous to the question of how much pain is felt. Furthermore, the simple paw-withdrawal technique has in fact demonstrated some consistency with human pain studies. In a recent pharmacological study that used a paw-withdrawal method in rodents with the SNL model (LaBuda and Little, 2005), hyperalgesia was reversed with gabapentin, amitriptyline, and fluoxetine, but not indomethacin, treatment. This correlates with the observation that the first three drugs are useful in treatment of neuropathic pain in humans but that nonsteroidal drugs are in general not useful.
A careful comparison of operant and reflex models in terms of their predictive capacity has yet to be performed. For drugs with a predominantly supraspinal action, operant measures may be more appropriate, whereas simple paw-withdrawal thresholds may be sufficient for drugs that work at peripheral or dorsal horn targets. Though the particulars of how pain is assessed remain an issue, a greater concern may relate to substantial inherent differences in the biology of nociception in rodent and man. Regardless of this, new drugs for treatment of neuropathic pain have been developed, and their utility in animal models played an influential role in moving these treatments into clinical trials.
Secondary Hyperalgesia and Central Sensitization
A starting place in understanding neuropathic pain is to consider what happens with injury to nonneural tissues. Skin injury produces ongoing pain and two types of hyperalgesia: primary and secondary (Meyer et al., 2006). Primary hyperalgesia occurs at the site of tissue injury and is mediated in part by sensitization of primary afferent nociceptors. This is reflected by increased responses to heat stimuli, for example. Secondary hyperalgesia occurs in the uninjured tissue surrounding the site of injury and is thought to be due to sensitization in the central nervous system. Secondary hyperalgesia is characterized by hyperalgesia to mechanical, but not heat, stimuli. This mechanical hyperalgesia is comparable to the hyperalgesia seen in patients with neuropathic pain. Two types of mechanical hyperalgesia are observed: pain to light-stroking stimuli (i.e., allodynia) and enhanced pain to punctate stimuli.
Two psychophysical studies in human volunteers provide strong evidence that secondary hyperalgesia is due to sensitization in the central nervous system. In both studies, intradermal injection of capsaicin, the algesic substance in hot peppers, was used to produce a large zone of secondary hyperalgesia. In the first study, the nerve supplying the area to be injected was anesthetized with a local anesthetic (LaMotte et al., 1991). Injection of capsaicin still produced a large flare, consistent with the concept that the flare reflects a peripherally mediated release of vasoactive peptides subsequent to antidromic spread of action potentials in the nociceptive terminals. In the opposite control arm, capsaicin injection at the same time produced intense pain and a large zone of secondary hyperalgesia. When the anesthesia wore off, no zone of mechanical hyperalgesia was found in the test arm, but hyperalgesia persisted in the control arm. This experiment provides evidence that the barrage of nociceptor activity associated with the capsaicin injection leads to an altered central processing of input from mechanosensitive afferents.
In the second study, a fine electrode was placed into the superficial peroneal nerve (Torebjörk et al., 1992). Electrical intraneural microstimulation produced a non-painful tactile percept that was referred to a small zone on the top of the foot. Capsaicin was injected adjacent to this area so that the zone of secondary hyperalgesia overlapped the area of referred sensation. Microstimulation after the injection produced a painful percept. Because this stimulation bypasses any peripheral sensitization processes at the cutaneous receptor, this experiment also provides evidence for altered central processing of mechanoreceptor input. Additional studies have shown that the tactile pain arises from central sensitization to the inputs of Aβ fibers, whereas the punctate hyperalgesia is due to a central sensitization to the inputs of capsaicin-insensitive Aδ nociceptors (Magerl et al., 2001).
The tactile fibers have known convergence onto dorsal horn cells that in addition receive inputs from nociceptive primary afferents. The inputs from the nociceptors in the injury zone are presumed to sensitize these so-called wide-dynamic range neurons and thereby enhance the synaptic efficacy of the tactile fibers. Nociceptive-specific neurons (dorsal horn neurons in lamina I) may be sensitized in similar fashion.
Central sensitization to the inputs of tactile afferents in patients has been demonstrated in patients with neuropathic pain. A blood pressure cuff inflated in the proximal extremity leads to an orderly loss of sensation beginning first with loss of tactile function. When this was done in patients, the loss of tactile function coincided with loss of allodynia (Campbell et al., 1988). It was also noted that the detection of pain with tactile stimuli was fast and indicated that primary afferents transmitting the stimulus were likely in the A-β range.
Where Do the Abnormal Signals in Neuropathic Pain Originate?
As shown in Figure 2, multiple sites along the neural axis are altered after nerve injury. Abnormalities occur in the injured and uninjured afferents supplying the affected region. Central sensitization, similar to that discussed above following tissue injury, is observed. In addition, changes in descending control systems have been reported. Finally, an immune response, both peripherally and centrally, is observed.
Figure 2. A Spinal Nerve Injury Leads to Alterations at Many Sites along the Neural Axis for Pain.
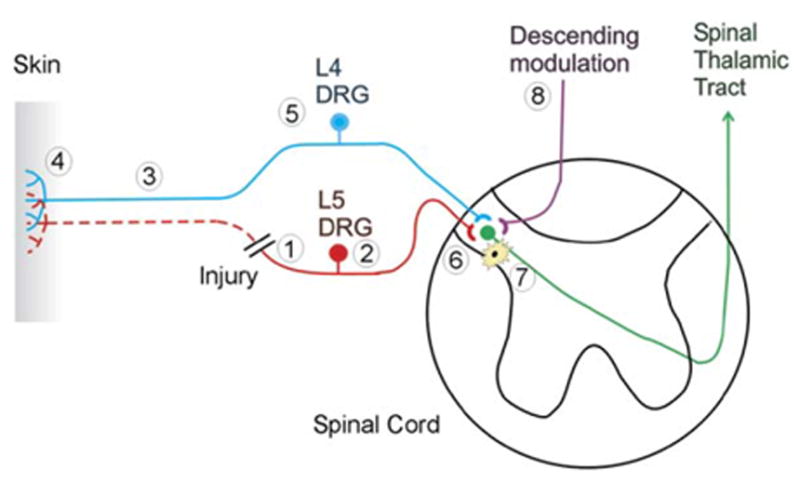
Eight different sites of pathophysiological changes are shown. (1) Spontaneous neural activity and ectopic sensitivity to mechanical stimuli develops at the site of nerve injury. (2) The expression of different molecules in the dorsal root ganglion of the injured nerve is up- or downregulated, reflecting the loss of trophic support from the periphery. Spontaneous neural activity develops in the dorsal root ganglia. (3) The distal part of the injured nerve undergoes Wallerian degeneration, exposing the surviving nerve fibers from uninjured portions of the nerve to a milieu of cytokines and growth factors. (4) Partial denervation of the peripheral tissues leads to an excess of trophic factors from the partly denervated tissue that can lead to sensitization of primary afferent nociceptors. (5) The expression of different molecules in the dorsal root ganglion of the uninjured nerve is up- or downregulated, reflecting the enhanced trophic support from the periphery. (6) Sensitization of the postsynaptic dorsal horn cell develops, leading to an augmentation of the response to cutaneous stimuli. (7) Activated microglial cells contribute to the development of this dorsal horn sensitization. (8) Changes in descending modulation of dorsal horn neurons also may contribute to the enhanced responsiveness of dorsal horn neurons.
A Role for Primary Afferents
The importance of primary afferent inputs in neuropathic pain is strongly suggested by several pharmacological studies. For example, systemic administration of AM1241, a selective CB2 cannabinoid receptor agonist, results in reversal of signs of mechanical and thermal hyperalgesia following an SNL lesion (Ibrahim et al., 2003). Because CB2 is not expressed in the central nervous system, the effects are likely due to a peripheral mechanism.
Systemic administration of artemin dose-dependently reversed the behavioral signs of neuropathic pain in rats with an SNL injury (Gardell et al., 2003). Artemin is a member of the glial-derived neurotrophic factor (GDNF) family that signals through a common tyrosine kinase receptor (RET) and GFRα3. GFRα3 is an accessory protein that is predominantly expressed in small-diameter, unmyelinated neurons (Orozco et al., 2001). The artemin-induced changes in neuropathic pain behavior are therefore most likely due to its action on nociceptive primary afferents.
Antisense oligodeoxynucleotides (ODNs) directed against Nav 1.8 reversed signs of mechanical hyperalgesia (Porreca et al., 1999). Nav 1.8 is a tetrodotoxin-resistant sodium channel that is primarily expressed in small-diameter primary afferent neurons. This treatment was effective even when applied 6–14 days after nerve injury, demonstrating that ongoing peripheral neuronal input is critically involved in the maintenance of neuropathic pain.
The Injured Afferent Hypothesis
The above evidence makes a clear case for the role of primary afferents, and one might assume that the culprit is the injured afferent itself. Indeed, much evidence favors this hypothesis. When nerve is injured a neuroma forms. The spontaneous activity and ectopic sensitivity to mechanical, thermal, and chemical stimuli that originate from the traumatic neuroma have been well documented (Blumberg and Jänig, 1984; Devor, 2006a). Clinical evidence has suggested that local-anesthetic blockade of an injured nerve in patients relieves pain, though no study, surprisingly, has really addressed this issue in a well-designed blinded fashion (Arner et al., 1990; Burchiel et al., 1993; Gracely et al., 1992; Koltzenburg et al., 1994). Additional support for the role of injured afferents comes from experiments in the rat L5 SNL model in which anesthetics (Sukhotinsky et al., 2004) or tetrodotoxin (TTX) (Lyu et al., 2000) directed at the L5 ganglia reversed the neuropathic behavior.
Regardless of the reasonableness of the so-called injured afferent hypothesis, several lines of evidence suggest that there is more to the story.
Surprisingly, several authors report that the L5 SNL model in rat leads to spontaneous activity in A fiber, but not C fiber, afferents as recorded in the L5 dorsal root (Boucher et al., 2000; Liu et al., 2000). This spontaneous activity in myelinated fibers appears to be produced mainly in afferents that (preinjury) serve muscle and joint, but not skin (Michaelis et al., 2000; Proske et al., 1995). These findings produce a dilemma because activity in C fibers is thought to be necessary to evoke the central sensitization mechanisms that account for the hyperalgesia (Ji and Woolf, 2001). One possible explanation is that A fiber-spontaneous activity can initiate central sensitization after nerve injury, if the afferent undergoes a phenotypic switch. A de novo expression of neuropeptides normally only expressed in nociceptive afferents could occur. For example, substance P immunoreactivity increases in large- and medium-size DRG neurons after axotomy (Noguchi et al., 1994). However, studies investigating substance P release from myelinated afferents following nerve injury are inconclusive (Allen et al., 1999; Malcangio et al., 2000). Another explanation is that the spontaneous activity occurs in “A-β” nociceptors, the existence of which has been documented in rodent and primate (Djouhri and Lawson, 2004; Treede et al., 1998).
Other behavioral data suggest that signals originating from the injured spinal nerve are not essential for hyperalgesia to occur. An L5 dorsal rhizotomy immediately before or after an L5 spinal nerve ligation did not prevent or reverse neuropathic behavior (Eschenfelder et al., 2000; Li et al., 2000). The counter to this observation is that dorsal rhizotomy by itself may produce signs of mechanical hyperalgesia (Colburn et al., 1999; Eschenfelder et al., 2000). More importantly, neuropathic pain behavior is observed after an L5 ganglionectomy, where the injured afferents are removed altogether (Sheth et al., 2002). The prevailing message seems to be that hyperalgesia can develop in the absence of neural activity from the injured nerve.
The Intact Nociceptor Hypothesis
According to this hypothesis, the intact nociceptors that survive injury and that innervate the region affected by the transected nerve fibers sensitize and have spontaneous activity. These changes in the intact nociceptors may induce ongoing pain and may account for certain aspects of hyperalgesia.
Following peripheral nerve lesions in primate and rodent models, spontaneous activity developed in uninjured, unmyelinated nociceptive afferents that shared the same innervation terrritory of the transected fibers (Ali et al., 1999; Djouhri et al., 2006; Wu et al., 2001). Although the average discharge frequency was low (seven action potentials/5 min), the incidence of spontaneously active fibers was high (50%). Low rates of spontaneous activity may therefore assume importance if this phenomenon is occurring in large numbers of C fibers, in particular given the high convergence of C fiber input in the CNS. Consistent with this hypothesis is the observation that low-frequency electrical stimulation in C fibers can lead to hyperalgesia in humans (Klede et al., 2003) and behavioral signs of hyperalgesia in rats (Wu et al., 2002). The development of spontaneous activity has also been observed in uninjured, myelinated afferents in the L4 dorsal root (Boucher et al., 2000), and this activity originated within the dorsal root ganglion.
Sensitization has also been reported in the intact nociceptors. Following peripheral or spinal nerve lesion in rat or rabbit, uninjured afferents develop adrenergic sensitivity (Sato and Perl, 1991) and an increased sensitivity to tumor necrosis factor-α (TNFα) (Schäfers et al., 2003). One study has suggested that sensitization to mechanical and heat stimuli occurs in the uninjured, unmyelinated afferents as well (Shim et al., 2005). Increased responsiveness to heat stimuli could be due to the observed increases in expression of mRNA and protein for the transient receptor potential receptor V1 (TRPV1) in the DRG of uninjured afferents (Fukuoka et al., 2000; Hudson et al., 2001). In addition, the incidence of cold-responsive neurons in the L4 DRG is increased after an L5 SNL (Djouhri et al., 2004), and there is an increase in expression of the putative cold-sensitive channel TRPA1 (Katsura et al., 2006). These observations may account for the occurrence of cooling hyperalgesia seen in patients after nerve trauma.
One compelling line of evidence in favor of the intact nociceptor hypothesis stems from experiments where local treatments are applied to the partly denervated skin. Local treatments can only affect the intact fibers. In patients, local treatment with capsaicin may significantly relieve ongoing pain. This is of interest because the effects of capsaicin are specific to nociceptors. Experiments with animal models point to similar conclusions. Mibefradil, a T-type calcium-channel blocker, reversed mechanical and thermal hyperalgesia (Dogrul et al., 2003), and local application of a glutamate receptor antagonist reversed signs of thermal hyperalgesia (Dogrul et al., 2000). In both studies, contralateral injections were without effects. Intraplantar injection of morphine on the ipsilateral, but not contralateral, side significantly reversed mechanical allodynia (Pertovaara and Wei, 2001).
The intact L4 dorsal root ganglion has been studied extensively to determine changes in phenotype after injury to the adjacent L5 spinal nerve (L5 SNL). In addition to increased expression of TRPV1 and TRPA1, mRNA for calcitonin gene-related peptide (CGRP) is upregulated (Fukuoka et al., 1998). Furthermore, the number of uninjured DRG neurons expressing brain-derived neurotrophic factor (BDNF) is increased (Fukuoka et al., 2001). In the SNI model, there is an upregulation of P2X3 mRNA in uninjured neurons (Tsuzuki et al., 2001).
Sympathetically Maintained Pain and the Intact Nociceptor Hypothesis
CRPS is a striking disorder manifest by severe pain typically in an extremity. Patients typically have edema, hyperalgesia, and may even have a motor disability that is difficult to explain from a purely electrophysiological perspective. In certain of these patients, selective anesthetic blockade of the sympathetic nervous system leads to dramatic relief of pain (sympathetically maintained pain, or SMP). Blockade of α -adrenergic receptor function with intravenous infusion of the antagonist phentolamine also leads to pain relief (Raja et al., 1991). In patients with SMP, in whom a sympathetic ganglion block was done to relieve pain and block release of norepinephrine, injection of physiological concentrations of norepinephrine evoked substantial pain (Figure 3A).
Figure 3. A Model for Sympathetically Maintained Pain.
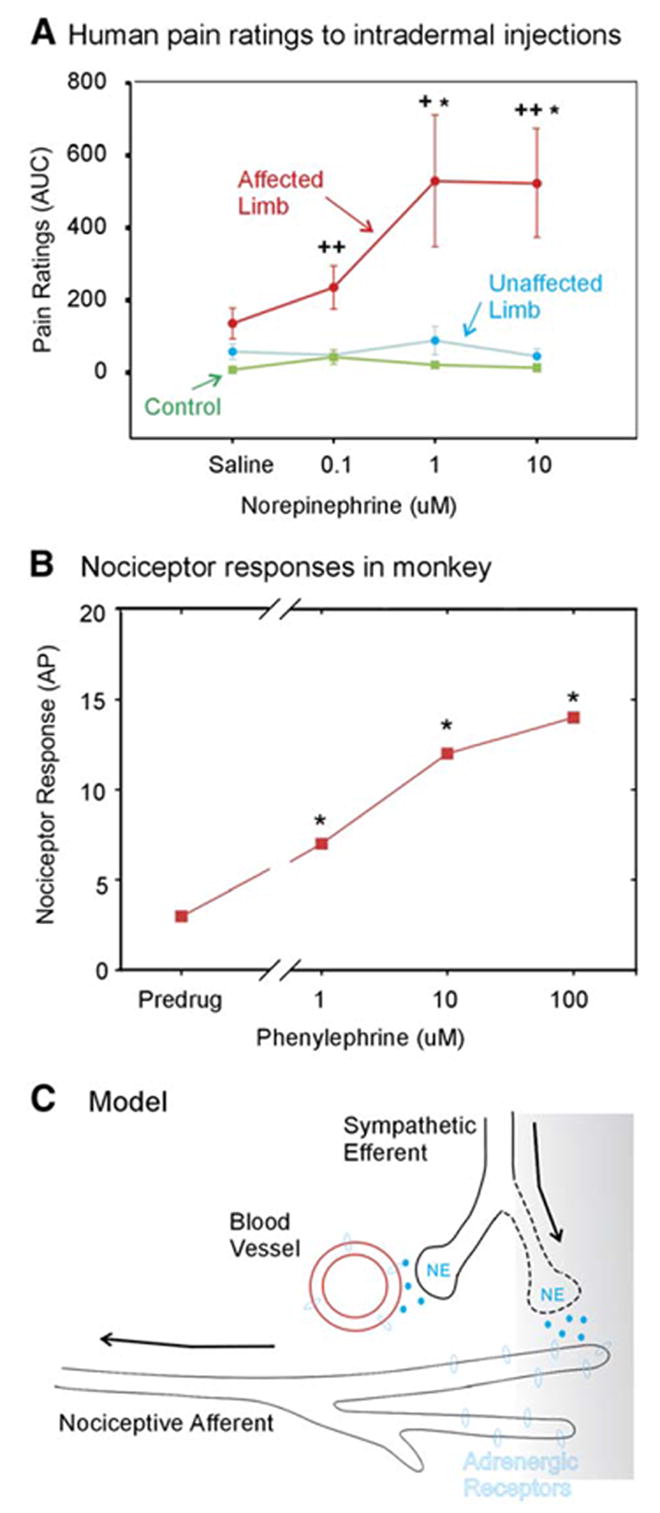
(A) After acutely relieving pain by performing a sympathetic block, norepinephrine in physiological concentrations was injected intra-dermally in a blinded fashion into the previously hyperalgesic area. The norepinephrine injections into the affected, but not the unaffected, extremity produced pain. Norepinephrine does not induce pain in control subjects. These data suggest that the nociceptors are sensitized to catechols in patients with SMP (adapted with permission [Ali et al., 2000]). ACUC, area under curve.
(B) In primates, normal nociceptors do not respond to catechol administration. However, in a monkey-spinal nerve ligation model, nociceptors developed a response to the α 1 adrenergic agonist phenylephrine administered topically to their receptive field (adapted and used with permission [Ali et al., 1999]). AP, action potentials. (C) A model to account for SMP. After a partial nerve lesion, some afferent fibers still remain in the skin. Factors released in the skin induce the sympathetic efferents to sprout into more superficial areas of the skin (Yen et al., 2006). These factors also lead the nociceptors to express α 1 adrenergic receptors. Now, the release of norepinephrine from the sympathetic terminals leads to activation of the nociceptive terminals, which accounts for the coupling of sympathetic activity with nociceptor responses.
Data are presented as mean ± SEM.
In a primate model of neuropathic pain, an L6 SNL leads to hyperalgesia just as it does in rodent models (Carlton et al., 1994). About one-half of the afferents to the top of the foot are lost, and the other half reach the foot from the adjoining and putatively normal spinal nerves (Ali et al., 1999). More than 60% of the intact nociceptors had spontaneous activity (normally infrequently seen), and more than 50% had sensitivity to the select α -adrenergic agonist phenylephrine (Figure 3B). These studies indicate that the mechanisms of SMP relate to an adrenergic sensitization of nociceptors (Figure 3C). Moreover, this chemical sensitization occurs in the intact nociceptors that survive a proximal nerve injury. Thus, SMP is a specific example of the importance of the intact nociceptors in the pathogenesis of a particular neuropathic pain disorder.
The Role of Growth Factors
Trophic factors in the target tissue have an ongoing influence on sensory and motor fibers. Nerve injury induces changes in growth-factor expression (Griffin, 2006). The change in expression occurs in the tissue deprived of innervation (e.g., the skin), the Schwann cells affected by Wallerian degeneration (denervated Schwann cells), the DRG, and the dorsal horn. Of note, in the SNL model, the L5 root is lesioned, but the L4 root shares the innervation territory, the same Schwann cells, and has convergent input to dorsal horn cells served by the L5 afferents. Nerve injury leads to increased levels of NGF in the skin supplied by L4. The binding of this NGF to the Trk-A receptors on the L4 fibers leads to transport of NGF back to the L4 DRG. Evidence suggests that this increased level of NGF in the L4 DRG affects factors such as BDNF. Indeed, the changes in gene expression in the intact L4 DRG and the lesioned L5 DRG are quite different.
Griffin has introduced the terms “undertrophed” and “overtrophed” to describe a putative mechanism by which growth factors may dually affect both the injured and uninjured afferents (Griffin, 2006). The injured axons by losing connectivity with the peripheral tissues lose trophic influences. The increased expression of trophic factors in the denervated tissues on the other hand “overtroph” the remaining (intact) fibers. NGF might be one of the villains in the case of overtrophing, whereas GDNF might be the missing growth factor, absence of which generates the pain-signaling mechanisms in the injured L5 spinal nerve (Boucher and McMahon, 2001).
Transduction, Channel Function, and Ectopic Discharge
Primary afferents transduce different forms of energy into generator potentials. If of sufficient magnitude, these generator potentials lead to action potentials. The action potentials propagate along the axon to the CNS. Pathological pain may result from disorders involved in any of these steps.
There are two ways nociceptors may be sensitized. One is for the transduction channel to sensitize (i.e., a bigger generator potential to a given natural stimulus). The other mechanism regards a decrease in threshold of the sodium channels responsible for spike initiation. It is logical therefore to ask whether primary afferent sensitization may account for features of neuropathic pain. NGF regulates TRPV1 expression, and thus, over-trophing mechanisms could link growth factors to transduction-channel function (Cortright and Szallasi, 2004). A role for TRPV4, a channel that may be involved in transduction of mechanical stimuli, was suggested in a neuropathic pain model produced by administration of taxol (Alessandri-Haber et al., 2004). Abnormal transduction, at least in part, likely underlies the pathophysiology of the “intact nociceptor.” Sensitization of the “intact” nociceptor in the SNL model may be responsible for the hyperalgesia (Ringkamp and Meyer, 2006). Another mechanism relates to the acquisition of transduction capacity for stimuli that ordinarily do not activate nociceptors. This has been clearly demonstrated in the case of SMP, where intact nociceptors acquire sensitivity to norepinephrine. Cooling hyperalgesia is prominent in neuropathic pain. Abnormal expression of transduction channels such as TRPA1, or TRPM8 in nociceptors, could account for this phenomenon.
Transduction can be displaced to a point of nerve injury as well. This misplaced transduction could account for the so-called “Tinel sign,” a useful clinical finding, wherein patients experience paresthesias (tingling sensation) or dysthesthesia (an unpleasant or frankly painful sensation) when the area over the nerve injury is tapped. The Tinel sign may be present at a point of entrapment where continuity of the axon has not necessarily been interrupted. The presumed mechanism is that a disturbance of fast axonal transport (which serves as delivery mechanism for newly synthesized transduction proteins) leads to ectopic expression of the transduction proteins. Axotomy leads to neuroma formation, where budding regenerative nerve sprouts devoid of growth guidance from denervated Schwann cells form an entangled mass. Many neuromas are “painful,” but for unclear reasons (genetic factors, location?) many are not. Areas of nerve injury may be tethered to adjacent moving structures (e.g., tendons), such that otherwise normal movements evoke an increase in pain presumably by activating nociceptive afferents through activation of mechanoreceptive transduction channels.
Nociceptor sensitization could also relate to changes in voltage-gated sodium channels, particularly in the region of spike initiation (Devor, 2006b). For example, if the threshold for sodium-channel activation decreases, the response to a given generator potential will be enhanced.
One reason for interest in sodium-channel function centers on clinical evidence that drugs that affect sodium-channel function may be very effective in relieving neuropathic pain. For example, the anticonvulsant carbamazepine, which works by stabilizing sodium channels in an inactive state, is considered the treatment of choice for trigeminal neuralgia. The effects are consistent enough that clinicians are trained to question the diagnosis if the patient does not have at least some response to the drug. As a class, each of the anticonvulsants that work through a sodium-channel mechanism (lamotrigine, phenytoin, in addition to carbamazepine) have some level of efficacy in neuropathic pain, though side effects often preclude usefulness. Another class of drugs that is effective in neuropathic pain is the local anesthetics (Tremont-Lukats et al., 2006). At doses that have no effect on normal sensibility, local anesthetics may favorably affect neuropathic pain.
There are multiple types of voltage-gated sodium channels that are expressed in the dorsal root ganglion and may have a role in neuropathic pain. The channels that have received greatest interest are Nav 1.3, 1.7, 1.8, and 1.9. These channels are found in small DRG cells and therefore are likely involved in action potential generation and conduction in nociceptors. Channels Nav 1.3 and 1.7 are TTX sensitive, whereas Nav 1.8 and 1.9 are TTX insensitive. Some animal data suggest that TTX given systemically may relieve hyperalgesia without adversely affecting normal functions (Marcil et al., 2006). Of the channels tested, only the TTX-sensitive channel Nav 1.3 is upregulated in the DRG of the injured axons (Black et al., 1999). This channel has kinetic properties that could favor repetitive spiking.
Whereas prior efforts have concentrated on the α -subunit expression, other complexities affect sodium-channel function. Expression of the β subunits could be part of this complexity. β2 subunits regulate channel gating, assembly, and cell-surface expression of TTX-sensitive channels. Expression of these subunits is increased in injured sensory afferents (Pertin et al., 2005), and in β2 null mice, mechanical hyperalgesia is reduced in a spared nerve injury model. β2 null mice display marked reduction in TTX-sensitive sodium current in small DRG neurons (Lopez-Santiago et al., 2006).
That channel function may strongly affect nociception has been further emphasized by the discovery of the genetic underpinnings of a striking neuropathic pain disorder known as erythromelalgia. Here, patients present with profound heat hyperalgesia and ongoing burning pain typically affecting the feet. The feet may also be intensely red, indicating profound vasodilation. Point mutations in the TTX-sensitive channel Nav1.7 account for the disorder (Figure 4). These mutations lead to a hypo-excitability in sympathetic neurons (accounting for the increased perfusion in the feet) and a hyperexcitability in small-sensory neurons (accounting for the pain and hyperalgesia) (Rush et al., 2006). Release of vasoactive peptides in the skin from the tonically active nociceptors may also contribute to the vasodilatation.
Figure 4. A Genetic Basis for Erythromelalgia.
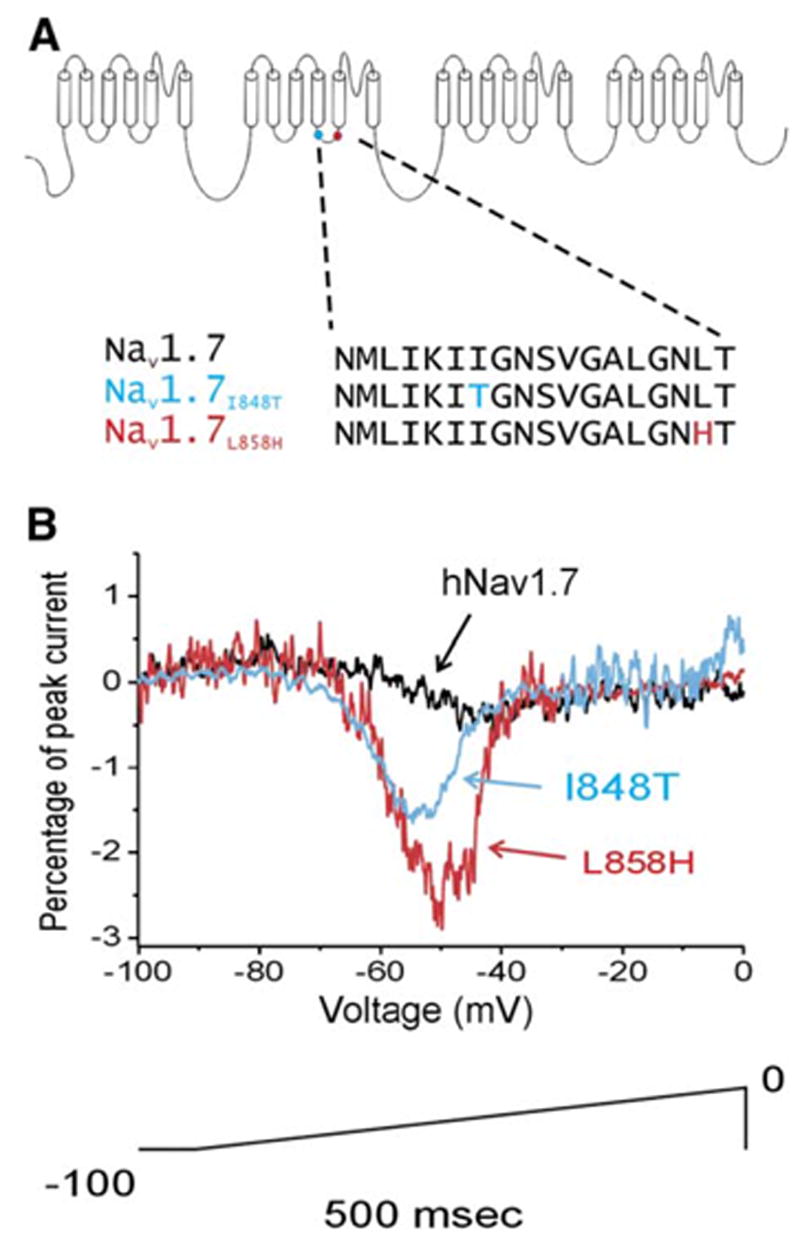
(A) Schematic of the Nav 1.7 voltage-gated sodium channel that is found exclusively in sensory and sympathetic neurons. A number of point mutations have been identified in this sodium channel in families of patients with erythromelalgia (Waxman and Dib-Hajj, 2005). The two mutations shown here produced single amino acid substitutions at the sites indicated.
(B) Whole-cell-patch-clamp recordings in transfected HEK293 cells revealed that these point mutations lead to an augmentation of the response of the channel to a slow depolarizing ramp (100 mV in 500 ms). These mutations would be expected to increase the excitability of the peripheral sensory neuron. (Adapted with permission [Cummins et al., 2004]. Copyright 2004 by the Society for Neuroscience.).
The Role of Central Sensitization
Central sensitization refers to the augmented response of central signaling neurons. Though thalamic and cortical levels may be involved, most attention has focused on the dorsal horn and in this review we will concentrate on this area.
When it occurs as a result of inflammation, central sensitization is strongly dependent on ongoing input from nociceptors. Thus, if nociceptive input is blocked in the injury zone, secondary hyperalgesia abates promptly (LaMotte et al., 1991). This same dependence on peripheral input probably also applies to central sensitization associated with neuropathic pain, but this issue requires further clarification. This distinction is of great importance clinically. If mechanisms of pain are completely centralized, it might be argued that attention to peripheral mechanisms is unlikely to be therapeutically beneficial.
Central sensitization involves homosynaptic and heterosynaptic mechanisms (Magerl et al., 1998; Woolf et al., 1988). Homosynaptic sensitization means that the conditioning stimulus and the test stimulus involve the same input. In dorsal horn nociceptive neurons, this is evident in a phenomenon referred to as “windup,” where continual low-frequency stimulation of C fiber afferents leads to an increasing response in the dorsal horn cell. Windup lasts tens of seconds and represents a short-term form of sensitization.
Herterosynaptic sensitization means that the conditioning stimulus and the test stimulus involve different sets of afferents. Heterosynaptic sensitization accounts for allodynia. Here, nociceptive inputs alter synaptic efficacy such that A-β mechanoreceptors acquire the capacity to evoke responses. Electrophysiological evidence for heterosynaptic sensitization has been shown in primate studies of dorsal horn cells where the response to light stroking of the skin was enhanced after capsaicin injection (Simone et al., 1991).
Ample evidence indicates that heterosynaptic and homosynaptic sensitization occur in neuropathic pain conditions (Campbell et al., 1988; Ji et al., 2003). Figure 5 provides a model for how this may occur in the L5 SNL model of neuropathic pain. Injured myelinated fibers from the L5 root develop spontaneous activity. Some of this activity could be in A-β mechanoreceptors (that have acquired the capacity to release substance P) or in A-δ nociceptors. The input of these fibers onto cells in the L5 segment could lead to homosynaptic sensitization and enhanced ongoing activity. The enhanced activity in L5 spinal thalamic tract cells could play a role in ongoing pain sensation. The injured L5 afferents could also project to the adjacent L4 segment where they could produce heterosynaptic sensitization to input from the L4 segment. This could account for the hyperalgesia to mechanical and heat stimuli that is signaled by activity in the intact L4 afferents.
Figure 5. Central Sensitization Mechanisms Involved in the Spinal Nerve Ligation Model.
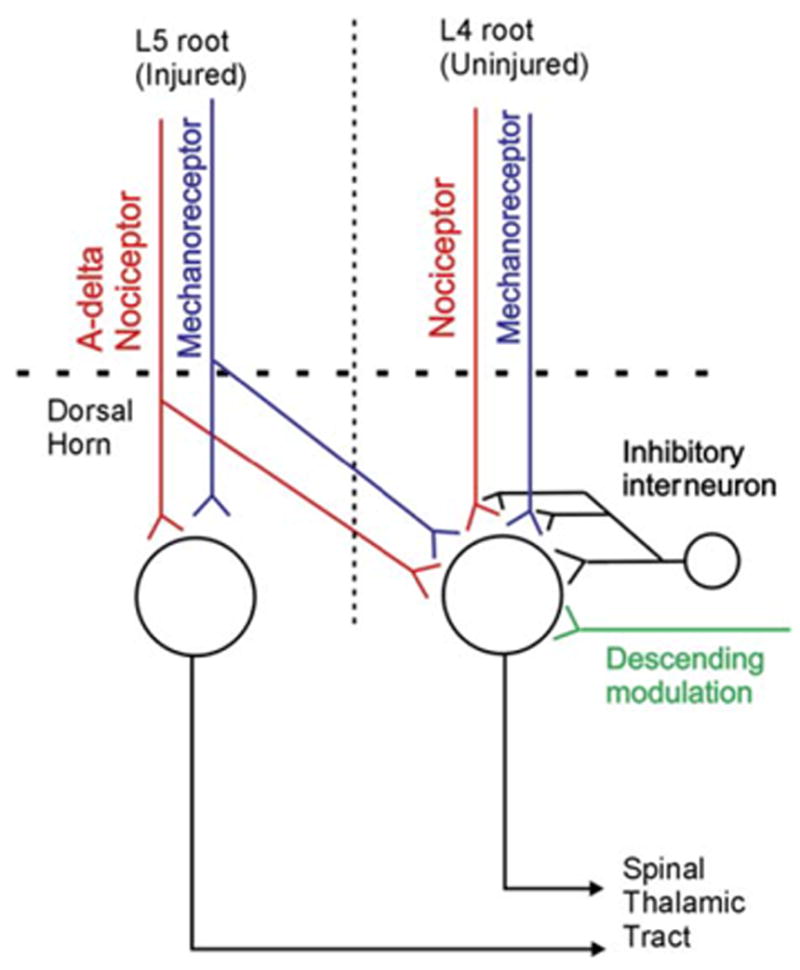
Spontaneous activity from the injured afferents (L5) and intact nociceptors (L4) may sensitize central pain-signaling neurons. The spontaneous activity in the L5 fibers is restricted to myelinated afferents. Nociceptive C fibers from L4 spontaneously discharge and may themselves be sensitized. The enhanced discharge of the primary afferents leads to augmented response of dorsal horn cells to nociceptor input and increased synaptic efficacy of inputs from mechanoreceptors (mechanism for allodynia). Alterations in descending modulation and inhibitory interneuron function also likely play a role.
Uninjured nociceptors in L4 root can develop spontaneous activity after an L5 SNL. This spontaneous activity can lead to homosynaptic sensitization such that stimulus-evoked activity in these nociceptors results in an augmented response of the dorsal horn cells. The spontaneous activity in uninjured L4 nociceptors could also produce heterosynaptic sensitization such that the response of L4 spinal thalamic cells to L4 mechanoreceptor input is enhanced. A brush stimulus applied to the foot activates the A-β fibers in the L4 root and acquires the capacity to drive activity in the L4 dorsal horn cell, consistent with heterosynaptic sensitization.
Homosynaptic and heterosynaptic sensitization involve many mechanisms, but fundamentally these mechanisms come down to two processes: (1) increased release of excitatory neurotransmitter (e.g., glutamate, substance P) and/or (2) enhanced synaptic efficacy. These changes in turn may relate to several cellular mechanisms (Basbaum, 1999). These mechanisms may be considered in five categories: (1) presynaptic changes, (2) postsynaptic changes, (3) interneuron changes, (4) changes in descending modulation, and (5) immune/microglial mechanisms (discussed in the following section). Evidence suggests a role for each of these mechanisms in neuropathic pain. Long-term potentiation (LTP), a phenomenon that has received intensive study in the hippocampus, appears to apply to the dorsal horn to some degree. However, given the abnormal ongoing input of primary afferents documented after nerve injury, the acute central-sensitizing effects of primary afferents may have an ongoing role. Thus, both short-term sensitization and LTP mechanisms likely apply. A role for long-term depression (LTD) in inhibitory cells is also considered below.
Presynaptic Mechanisms
Release of glutamate is inhibited presynaptically by several metabotropic G protein-coupled receptors, including μ-opioid receptors, GABA-b, and adenosine receptors. Downregulation of μ-opioid receptors has been documented both pre- and postsynaptically after nerve injury (Kohno et al., 2005) and could lead to an enhanced release of glutamate.
Neurotransmitter release is triggered by voltage-gated calcium channel activity on the central terminals of primary afferents. Nerve injury leads to an upregulation of the α -2-δ subunit of these channels in the DRG and spinal cord (Li et al., 2004). This could be associated with increased calcium entry and augmented release of glutamate. The anticonvulsant drugs gabapentin and pregabalin are effective in neuropathic pain presumably because they bind to and block this subunit (Bian et al., 2006; Freynhagen et al., 2005). Although these drugs reverse hyperalgesia in neuropathic models, there is no apparent effect on normal pain sensibility (Suzuki et al., 2005). This argues for a special role of the α -2 subunit in neuropathic pain.
There is also evidence to suggest that under conditions of nerve injury, A-β fibers undergo a phenotypic switch, such that they begin to express substance P (Noguchi et al., 1994). As a consequence, stimulation of these fibers leads to substance P release, providing an additional potential mechanism for sensitization of dorsal horn neurons.
Postsynaptic Mechanisms
There is excellent documentation of the role of postsynaptic mechanisms in central sensitization. Slow depolarization induced by substance P and other peptides leads to an opening of the NMDA glutamate-gated channel, which in turn leads to calcium entry (Dougherty et al., 1993; Duggan, 1995; Yoshimura and Yonehara, 2006). Calcium may gain entry through AMPA receptors that do not contain the GluR2 subunit, as well, and this mechanism may play a role in dorsal horn LTP (Gu et al., 1996). A conditional knockdown of one NMDA subunit, NR1, abolished hyperalgesia to formalin but had no effect on normal pain sensibility (South et al., 2003). Evidence exists for a multitude of intracellular events linked to postsynaptic sensitization that result from calcium entry. Protein kinases A and C and other transcriptional factors likely play important roles in these events (Kawasaki et al., 2004).
Another possible mechanism of postsynaptic sensitization involves an increased trafficking of AMPA receptors to the cell surface. The expression of AMPA receptors is increased in the superficial dorsal horn after an SNL lesion (Harris et al., 1996). In addition, upregulation of the mRNAs for both AMPA and NMDA receptors has been demonstrated in diabetes (Tomiyama et al., 2005).
We have suggested that allodynia stems from sensitization to the inputs of tactile afferents in the dorsal horn. However, certain evidence suggests that the “Aβ sensitization” phenomenon may actually arise from alternative pathways. A lesion of the ipsilateral dorsal column abolished mechanical hyperalgesia but, interestingly, not heat hyperalgesia (Ossipov et al., 2002; Sun et al., 2001). These data suggest that dorsal horn trafficking may account for heat hyperalgesia but that other centers, such as the thalamus, may actually account for the allodynia.
Role of Disinhibition Mechanisms
Inhibitory neurons play an important role in governing the sensitivity of dorsal horn neurons (Gu et al., 1996). Decreased expression of inhibitory receptors (on primary afferent terminals and postsynaptic neurons) has been noted following nerve injury, and sensitization could result from this disinhibition mechanism (Kohno et al., 2005). Several other mechanisms involving inhibitory interneurons have been suggested. We have discussed LTP. The reciprocal phenomenon is LTD. Salter has suggested that LTD may be evoked by activation of NMDA receptors present on GABAergic cells due to differential expression of the subunits in the NMDA receptor (Salter, 2006). LTD in inhibitory cells may lead to central sensitization (Randic et al., 1993). To what extent this mechanism applies to the dorsal horn is unclear.
Nerve injury also induces a downregulation of the potassium-chloride transporter KCC2. This trans-synaptic effect on superficial dorsal horn cells (presumed projection neurons of the spinothalamic tract), leads to a less-negative equilibrium potential for chloride, such that opening the chloride channel with GABA induces depolarization sufficient to induce excitation. Consistent with this finding, knockdown of the expression of KCC2 leads to hyperalgesia (Coull et al., 2003).
Further evidence indicates that nerve injury induces a selective apoptosis of inhibitory GABAergic inter-neurons (Moore et al., 2002). Apoptosis of inhibitory GABAergic neurons argues for a hard-wire change in circuitry (Scholz et al., 2005). This is a critical therapeutic issue. If central sensitization evolves such that nociceptive signaling becomes independent of inputs from primary afferents, then treating the peripheral nerve may no longer be therapeutically useful. Moreover, the relative importance of peripheral and central mechanisms may well depend on the underlying disease. Some diseases may involve primary afferents as well as the CNS. An example is postherpetic neuralgia where damage may extend from the primary afferents to the dorsal horn.
Descending Modulatory Mechanisms
Descending modulatory pathways also appear to influence dorsal horn sensitization mechanisms in neuropathic pain. Cortical, thalamic, and periaqueductal inputs converge on the rostroventromedial medulla (RVM). This center gives rise to both inhibitory and excitatory inputs to the dorsal horn via an ipsilateral pathway in the dorsolateral funiculus (DLF). Many of the cells in the RVM express μ-opioid receptors. Selective ablation of these cells either before or after establishment of the SNL model can eliminate hyperalgesia (Porreca et al., 2001). Lidocaine microinjection (Burgess et al., 2002) and hemisection experiments (Bian et al., 1998) have yielded similar results.
Supraspinal Mechanisms
Recent fMRI studies reveal changes in the processing of cutaneous stimuli at supraspinal centers in conditions of hyperalgesia and allodynia. Experimental studies of secondary hyperalgesia allow the comparison of fMRI images before and after the development of hyperalgesia. In these studies, mechanical stimulation of the skin produced enhanced pain in the zone of secondary hyperalgesia and led to enhanced activation in areas of the brain associated with pain signaling (Baron et al., 1999; Maihofner et al., 2004). Whether these changes in supraspinal processing are passive or whether they reflect additional plasticity mechanisms is difficult to know. Functional imaging studies in patients with neuropathic pain reveal that light stroking that produces pain recruits a complex cortical network, including nociceptive, motor, and cognitive areas (Maihofner et al., 2006; Schweinhardt et al., 2006). Functional imaging may lead to new insights into the mechanisms of neuropathic pain as well as to objective measures of altered sensations (Borsook et al., 2004).
The Immune System and Neuropathic Pain
Clearly the immune system is critical to the pain associated with inflammatory pain. The immune system appears to play a critical role in neuropathic pain as well (Marchand et al., 2005). Nude rats that lack mature T cells had diminished hyperalgesia (Moalem et al., 2004). Passive transfer of type 1, but not type 2, T cells led to similar levels of hyperalgesia as those seen in rats with intact T cell function. In addition, cytokines, II-1, II-6, and TNFα all have demonstrated roles in neuropathic pain (Manning, 2006). Several variables are worth noting in approaching the question of immune mechanisms.
One must distinguish the immune mechanisms associated with a particular disease from the immune mechanisms that are particular to the issue of neuropathic pain.
Immune mechanisms need to be considered separately in terms of what happens in the peripheral nervous system versus those that are important in the central nervous system.
Finally, immune mechanisms may play a role in initiating neuropathic pain, maintaining neuropathic pain acutely, and/or play a role in chronic neuropathic pain.
Peripheral Immune Mechanisms
Nociceptors have a rich variety of immune receptors, and evidence exists for roles of the interleukins (IL-1β and IL-6), TNFα, bradykinin, prostanoids, and others (Opree and Kress, 2000). In the CCI model of neuropathic pain, a “neuritis” develops, likely reflecting the inflammatory response to the suture material (Kleinschnitz et al., 2004). In this model, immune mechanisms might be a particular issue simply because of the foreign suture material. A more interesting question is what happens with simple surgical axotomy (e.g., the SNL model).
Nerve damage evokes a cascade of immune responses. Nerve damage leads to macrophage infiltration, T cell activation, and increased expression of proinflammatory cytokines. With axotomy, Schwann cells are in a sense denervated. These denervated Schwann cells (as well as cells in the partially denervated target tissue) may communicate with intact fibers that pass within the same nerve. This provides a mechanism by which to explain changes in the intact nociceptor (see above). Schwann cell denervation recruits macrophages via secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 (MCP-1) (Sugiura et al., 2000). MCP-1 serves as a ligand for CCR2 (Tofaris et al., 2002). CCR2 knockout mice do not develop mechanical hyperalgesia after a partial nerve ligation injury (Abbadie et al., 2003). Cytokine IL-1β leads to increased expression of nerve growth factor (NGF), and NGF may sensitize nociceptors (Kanaan et al., 1998). Knockout of the IL-1 receptor type I and genetically mediated overexpression of an IL-1 receptor antagonist decreased hyperalgesia. That the effect was peripheral was supported by the observation that spontaneous activity recorded from the dorsal root fibers was reduced (Wolf et al., 2006).
TNFα has received much attention as a culprit in neuropathic pain. Endoneurial administration induces hyperalgesia (Wagner and Myers, 1996). Preemptive treatment with the TNF-sequestering drug etanercept decreases hyperalgesia but has no effect once hyperalgesia is established (Sommer et al., 2001). TNFα can initiate activity in nociceptors (Sorkin et al., 1997). Inhibition of TNFα blocks phosphorylation of the MAP kinase p38 in DRG and hyperalgesia but again only when given preemptively (Schäfers et al., 2003). Another cytokine that has received attention is IL-6. A role in the CCI model has been suggested (Okamoto et al., 2001), but other peripheral actions are unclear at present.
Central Immune Mechanisms
Somewhat surprising is the mounting evidence that central immune mechanisms play a role in neuropathic pain (Figure 6) (Watkins and Maier, 2005). As with peripheral mechanisms, much evidence supports the view that initiation, but not maintenance, of neuropathic pain behaviors is associated with immune mechanisms (Xu et al., 2006). Microglia serve as the macrophages of the CNS. Nerve injury distal to the dorsal root ganglia leads to microglial activation. However, activation after dorsal rhizotomy is much less, in keeping with the observation that dorsal rhizotomy leads to less-impressive hyperalgesia compared to spinal nerve lesions involving the identical root (Willis et al., 2006). Interthecal delivery of the antibiotic minocycline, an inhibitor of microglial activation, attenuates neuropathic pain (Raghavendra et al., 2003).
Figure 6.
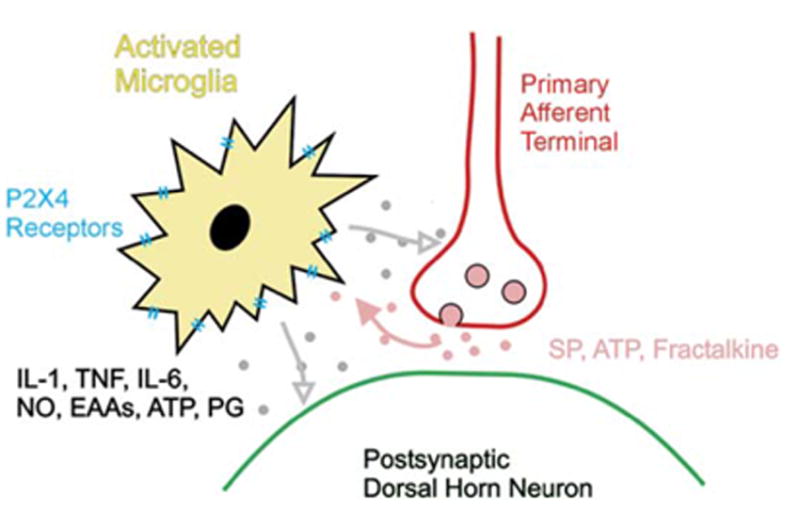
Microglial Cells in the CNS Are Activated following Peripheral Nerve Injury and Release Cytokines that Alter the Responses of Dorsal Horn Cells
The mechanisms are far from clear. Nociceptor activity leads to rapid activation of the MAP kinase ERK in microglia and initially not in other central cells (Tsuda et al., 2005). Inhibition of this activation attenuates pain behavior. The mechanisms for this activation might be multi-factorial. Evidence exists for a role of the ATP receptors P2X4 and/or P2X7, both of which are expressed on microglia. The chemokine fractalkine may also be involved, as blockade of its receptor, CX3CR1, attenuates hyperalgesia in neuropathic pain models (Milligan et al., 2005). The activated microglia produce the toxic cytokines IL-1, IL-6, and TNFα as well as nitric oxide, excitatory amino acids, ATP, and prostaglandins (Inoue, 2006).
The toll-like receptor-4 (TLR4) is a molecule involved in the innate immune response and is mainly expressed on microglia. Spinal mRNA for TLR4 is increased after L5 SNL. A knockout or point mutation in TLR4, as well as downregulation through intrathecal delivery of anti-sense ODN, has a significant effect on neuropathic pain behavior, but only when given in the early stages (Tanga et al., 2005).
Conclusion
We began this review by presenting a patient with chest-wall pain following a mastectomy. The simultaneous finding of decreased sensation and ongoing pain was suggested to be a paradox. Animal models, as well as studies in humans, however, have taken us a long way toward understanding this case of neuropathic pain. Ongoing pain likely represents spontaneous discharge in afferents. The injured afferents are an obvious source of abnormal input.
Sodium-channel dysfunction likely plays an important role in leading to ectopic generation of action potentials. The patient had episodes of abrupt “pain attacks.” These attacks may correspond to bursts of spontaneous activity in the injured afferents related to sodium-channel dysfunction. Somewhat surprisingly, however, the intact afferents that share the innervation territory of the injured afferents also discharge spontaneously. Studies of their cell bodies in the DRG reveal striking phenotype changes. Trophic factors released from the partly denervated skin working through their receptors on the peripheral terminals may account for these abnormalities. The abnormalities seen in these “intact” nociceptors likely account for the fact that patients such as this may respond to therapies applied at the level of the skin. Central changes also play a role in neuropathic pain. Many of the mechanisms probably are the same as those observed with inflammatory pain. The patient had pain and hyperalgesia well outside the region of nerve injury. This is precisely the case in inflammatory pain, where secondary hyperalgesia extends well beyond the injury site. The patient had substantial pain when the skin was lightly stroked (allodynia). Multiple lines of evidence indicate that this allodynia is due to central sensitization, such that tactile afferents acquire synaptic efficacy, which enables them to trigger activity in central pain signaling neurons. Prevailing work has focused on the dorsal horn as the site for this sensitization, but more rostral pathways may be involved as well. Though not easily understood as yet, peripheral nerve injury induces some striking transynaptic effects. One is apoptosis that appears to preferentially affect GABA inhibitory cells. Several lines of evidence indicate that immune mechanisms are involved both peripherally and centrally. Activation of microglial cells occurs in the dorsal horn, and this activation may play a vital role in initiating central sensitization. The role of this activation in ongoing neuropathic pain is less clear. The sensation of pain begins with a simple thesis: nociceptors encode information about noxious stimuli and propagate these messages to the CNS and pain is felt. In the case of neuropathic pain, however, we see that a rich biology is at play. Studies are quickly uncovering the mechanisms, and no longer can we consider the presence of this problem quite so mysterious.
Acknowledgments
We greatly appreciated the suggestions from Drs. Jasenka Borzan, Michael Caterina, and Matthias Ringkamp on earlier drafts of this manuscript and the technical assistance of Cheryl Carmona. This research was supported by the National Institutes of Health (NS-014447 and NS-041269) and the Blaustein Pain Treatment Center.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren SC, Levine JD. Mechanical hyperalgesia in streptozotocin-diabetic rats. Neuroscience. 1993;52:1049–1055. doi: 10.1016/0306-4522(93)90551-p. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha adrenergic sensitivity following L6 spinal nerve ligation in the monkey. J Neurophysiol. 1999;81:455–466. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–168. doi: 10.1016/S0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Arner S, Lindblom U, Meyerson BA, Molander C. Prolonged relief of neuralgia after regional anesthetic blocks. A call for further experimental and systematic clinical studies. Pain. 1990;43:287–297. doi: 10.1016/0304-3959(90)90026-A. [DOI] [PubMed] [Google Scholar]
- Baron R, Baron Y, Disbrow E, Roberts TP. Brain processing of capsaicin-induced secondary hyperalgesia: a functional MRI study. Neurology. 1999;53:548–557. doi: 10.1212/wnl.53.3.548. [DOI] [PubMed] [Google Scholar]
- Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci USA. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bushnell MC, Campbell JN, Chaplan SR, Mantyh PW, Porreca F, Price DD, Urban L, Vierck CJ, Zubieta JK. Measurrement and new technologies: rapporteur report. In Emerging Strategies for the Treatment of Neuropathic. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 361–381. [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bian D, Ossipov MH, Zhong C, Malan TP, Jr, Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal tansection in rats with nerve injury. Neurosci Lett. 1998;241:79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Jänig W. Discharge pattern of afferent fibers from a neuroma. Pain. 1984;20:335–353. doi: 10.1016/0304-3959(84)90111-8. [DOI] [PubMed] [Google Scholar]
- Boivie J. Central pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. London: Elsevier; 2006. pp. 1057–1074. [Google Scholar]
- Boivie J, Leijon G, Johansson I. Central post-stroke pain–a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989;37:173–185. doi: 10.1016/0304-3959(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Borsook D, Burstein R, Becerra L. Functional imaging of the human trigeminal system: opportunities for new insights into pain processing in health and disease. J Neurobiol. 2004;61:107–125. doi: 10.1002/neu.20085. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, McMahon SB. Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol. 2001;1:66–72. doi: 10.1016/s1471-4892(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of C-fos-like protein as a marker for neuronal activity following nixious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78:714–719. doi: 10.3171/jns.1993.78.5.0714. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN. Nerve lesions and the generation of pain. Muscle Nerve. 2001;24:1261–1273. doi: 10.1002/mus.1143. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Lekan HA, Kim SH, Chung JM. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain. 1994;56:155–166. doi: 10.1016/0304-3959(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Cook AW, Browder EJ. Function of Posterior columns in man. Arch Neurol. 1965;12:72–79. doi: 10.1001/archneur.1965.00460250076009. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Waxman SG. Electro-physiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24:8232–8236. doi: 10.1523/JNEUROSCI.2695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Devor M. Sensory basis of autotomy in rats. Pain. 1991;45:109–110. doi: 10.1016/0304-3959(91)90174-V. [DOI] [PubMed] [Google Scholar]
- Devor M. Response of nerves to injury in relation to neuropathic pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. London: Elsevier; 2006a. pp. 905–927. [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006b;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Wrigley D, Thut PD, Gold MS. Spinal nerve injury increases the percentage of cold-responsive DRG neurons. Neuroreport. 2004;15:457–460. doi: 10.1097/00001756-200403010-00015. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105:159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Zorn S, Willis WD. Combined application of excitaory amino acids and substance P produces long-lasting changes in responses of primate spinothalamic tract neurons. Brain Res Brain Res Rev. 1993;18:227–246. doi: 10.1016/0165-0173(93)90003-i. [DOI] [PubMed] [Google Scholar]
- Duggan AW. Release of neuropeptides in the spinal cord. Prog Brain Res. 1995;104:197–223. doi: 10.1016/s0079-6123(08)61792-6. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Dyck PJ, Larson TS, O’Brien PC, Velosa JA. Patterns of quantitative sensation testing of hypoesthesia and hyperalgesia are predictive of diabetic polyneuropathy: a study of three cohorts. Nerve growth factor study group. Diabetes Care. 2000;23:510–517. doi: 10.2337/diacare.23.4.510. [DOI] [PubMed] [Google Scholar]
- Elias WJ, Burchiel KJ. Microvascular decompression. Clin J Pain. 2002;18:35–41. doi: 10.1097/00002508-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Eschenfelder S, Häbler HJ, Jänig W. Dorsal root section elicits signs of neuropathic pain rather than reversing them in rats with L5 spinal nerve injury. Pain. 2000;87:213–219. doi: 10.1016/S0304-3959(00)00285-2. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Noguchi K. The role of neighboring intact dorsal root ganglion neurons in a rat neuropathic pain model. In: Devor M, Rowbotham M, Wiesenfeld-Hallin Z, editors. Progress in Pain Research and Management. Seattle, WA: ISAP Press; 2000. pp. 137–146. [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Griffin JW. The roles of growth factors in painful length-dependent axonal neuropathies. In Emerging Strategies for the Treatment of Neuropathic. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 271–290. [Google Scholar]
- Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes GM. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. [Google Scholar]
- Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kanaan SA, Poole S, Saade NE, Jabbur S, Safieh-Garabedian B. Interleukin-10 reduces the endotoxin-induced hyperalgesia in mice. J Neuroimmunol. 1998;86:142–150. doi: 10.1016/s0165-5728(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Klede M, Handwerker HO, Schmelz M. Central origin of secondary mechanical hyperalgesia. J Neurophysiol. 2003;90:353–359. doi: 10.1152/jn.01136.2002. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Brinkhoff J, Zelenka M, Sommer C, Stoll G. The extent of cytokine induction in peripheral nerve lesions depends on the mode of injury and NMDA receptor signaling. J Neuroimmunol. 2004;149:77–83. doi: 10.1016/j.jneuroim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Torebjörk HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Little PJ. Pharmacological evaluation of the selective spinal nerve ligation model of neuropathic pain in the rat. J Neurosci Methods. 2005;144:175–181. doi: 10.1016/j.jneumeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EFP. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Lau FH, Chung KC. Silas Weir Mitchell, MD: the physician who discovered causalgia. J Hand Surg [Am] 2004;29:181–187. doi: 10.1016/j.jhsa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci. 2006;26:7984–7994. doi: 10.1523/JNEUROSCI.2211-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74:257–268. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Schmelz M, Forster C, Neundorfer B, Handwerker HO. Neural activation during experimental allodynia: a functional magnetic resonance imaging study. Eur J Neurosci. 2004;19:3211–3218. doi: 10.1111/j.1460-9568.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66:711–717. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–399. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- Manning D. The role of neuroimmune activation in chronic neuropathic pain and new targets for therapeutic intervention. In Emerging Strategies for the Treatment of Neuropathic. In: Basbaum AI, Campbell JN, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 161–192. [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br J Anaesth. 2006;96:761–768. doi: 10.1093/bja/ael096. [DOI] [PubMed] [Google Scholar]
- Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993;54:57–69. doi: 10.1016/0304-3959(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. London: Elsevier; 2006. pp. 3–34. [Google Scholar]
- Michaelis M, Liu X, Jänig W. Axotomized and intact muscle afferents but not skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129:767–777. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a sub-population of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertin M, Ji RR, Berta T, Powell AJ, Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A, Wei H. Peripheral effects of morphine in neuropathic rats: role of sympathetic postganglionic nerve fibers. Eur J Pharmacol. 2001;429:139–145. doi: 10.1016/s0014-2999(01)01315-2. [DOI] [PubMed] [Google Scholar]
- Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci USA. 1999;96:7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the muopioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Iggo A, Luff AR. Mechanical sensitivity of regenerating myelinated skin and muscle afferents in the cat. Exp Brain Res. 1995;104:89–98. doi: 10.1007/BF00229858. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698. doi: 10.1097/00000542-199104000-00012. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M, Meyer RA. Does peripheral sensitization of primary afferents play a role in neuropathic pain? In Emerging Strategies for the Treatment of Neuropathic. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 87–102. [Google Scholar]
- Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc Natl Acad Sci USA. 2006;103:8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW. Signaling pathways in pain neuroplasticity in the spinal dorsal horn. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Emerging Strategies for the Treatment of Neuropathic Pain. Seattle, WA: IASP Press; 2006. pp. 193–209. [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Scadding JW, Koltzenburg M. Painful peripheral neuropathies. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. London: Elsevier; 2006. pp. 973–999. [Google Scholar]
- Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32:256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sheth RN, Dorsi MJ, Li Y, Murinson BB, Belzberg AJ, Griffin JW, Meyer RA. Mechanical hyperalgesia after an L5 ventral rhizotomy or an L5 ganglionectomy in the rat. Pain. 2002;96:63–72. doi: 10.1016/s0304-3959(01)00429-8. [DOI] [PubMed] [Google Scholar]
- Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: Central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-α induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, et al. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton-Hicks M, Janig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–133. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 2000;12:457–466. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Ben Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain. 2004;8:135–143. doi: 10.1016/S1090-3801(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LER, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- Tremont-Lukats IW, Hutson PR, Backonja MM. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22:266–271. doi: 10.1097/01.ajp.0000169673.57062.40. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- Vierck CJ. Animal studies of pain: lessons for drug development. In Emerging Strategies for the Treatment of Neuropathic. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 475–496. [Google Scholar]
- Vierck CJ, Jr, Light AR. Effects of combined hemotoxic and anterolateral spinal lesions on nociceptive sensitivity. Pain. 1999;83:447–457. doi: 10.1016/S0304-3959(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11:555–562. doi: 10.1016/j.molmed.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Hammond DL, Dubner R, Merzenich M, Eisenach JC, Salter MW, Iyengar S, Shippenberg T. Central nervous system targets: rapporteur report. In Emerging Strategies for the Treatment of Neuropathic. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Pain. Seattle, WA: IASP Press; 2006. pp. 105–122. [Google Scholar]
- Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW, King AE. Prolonged primary afferent induced alterations in dorsal horn neurones, an intra-cellular analysis in vivo and in vitro. J Physiol (Paris) 1988;83:255–266. [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Yen LD, Bennett GJ, Ribeir-oda-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Yonehara N. Alteration in sensitivity of ionotropic glutamate receptors and tachykinin receptors in spinal cord contribute to development and maintenance of nerve injury-evoked neuropathic pain. Neurosci Res. 2006;56:21–28. doi: 10.1016/j.neures.2006.04.015. [DOI] [PubMed] [Google Scholar]


