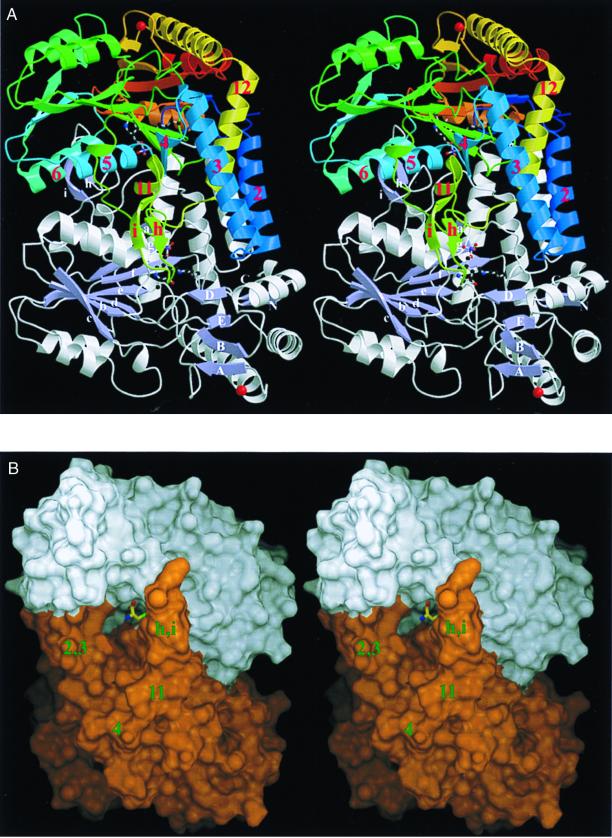
Figure 1.
Overall fold of C-DES. (A) Stereo ribbon drawing of the homodimer showing the PLP covalently bound to Lys-223 (color-coded by atom type) and the K+ ion (red) rigidifying a solvent-exposed loop in ball-and-stick mode. In the upper monomer, the course of the polypeptide chain is illustrated by a color ramp starting at the N terminus with blue and ending at the C terminus with red. Secondary structure elements, which are substantial for dimer stabilization, are labeled in red. The lower monomer is colored by secondary structure with the nomenclature of the two central β-sheets given. (B) Stereo representation of the surface of the active dimer color-coded by the monomers (white and orange). The product of cystine cleavage, cysteine persulfide, is shown in a ball-and-stick representation to illustrate the large dimensions of the active-site funnel. Note the enclosure of the terminal persulfidic group by C-DES to shield it from solvent. Fig. 1A was produced with molscript (23) and raster3d (24); all other figures were created with dino (www.bioz.unibas.ch/∼xray/dino).