Abstract
Primary care physicians may be directly or indirectly involved in the management of the bipolar patient. Bipolar affective illness is a chronic, recurrent disorder. Patients, their families and support systems, and the general public can face profound and enduring consequences if the illness is untreated or poorly treated. Consequently, increasing attention has been directed at developing treatment strategies to control symptoms associated with bipolar disorder. While lithium has been the mainstay of treatment for many years, recent investigations have demonstrated the utility of a number of anticonvulsant medications in bipolar disorder. This review will discuss the literature on anticonvulsant efficacy in bipolar disorder in light of the treatment guidelines set forth by the Bipolar Treatment Expert Consensus Panel and the American Psychiatric Association. To orient the clinician, issues related to anticonvulsant use, dosing, adverse effects, and drug interactions are also discussed.
Primary care physicians are increasingly at the forefront of assessing and managing affective disorders, e.g., depression and bipolar disorders. A prospective evaluation of patients endorsing depressed and/or anxious symptoms in a family practice outpatient setting found that 25% of the cohorts had a bipolar spectrum disorder.1 These data suggest that primary care physicians may be the first to encounter a patient with bipolar disorder. Effective management of the patient with a major mood disorder often requires a collaborative effort between the primary care physician and psychiatrist. The primary care physician may be instrumental in ensuring psychiatric evaluation and follow-up, may be the first to recognize early signs of recurrence, may detect noncompliance, and may be the primary source to whom patients present when adverse effects of use of mood-stabilizing agents are encountered. This article is written to inform primary care physicians about recent trends in the management of bipolar disorder. In addition to familiarizing the reader with bipolar disorder and its variants, the intent here is to heighten awareness of various treatment alternatives including dosing, potential adverse effects, risks associated with pregnancy and lactation, and possibilities of drug interactions.
DIAGNOSIS AND COURSE
Bipolar disorder is characterized by cycles of manic and depressive episodes. During a “classic” manic episode, the patient's mood is notably elated, euphoric, or irritable for a sustained period of at least 1 week's duration.2 This is accompanied by at least 3 additional symptoms, including inflated or grandiose self-esteem, decreased need for sleep, increased activity characterized by overt agitation or pursuing goal-directed activities, excessive pursuit of pleasurable activities with potentially dangerous consequences, talking more than usual with rapid and pressured speech, overt distractibility, and flight of ideas or a subjective sense that one's thoughts are racing.2 Often, patients who are in a manic episode have significantly impaired judgment and can lose touch with reality, i.e., the patient may have delusional beliefs or hallucinations. Manic patients may act impulsively on their beliefs or on poorly thought out plans, for example, taking on tasks that cannot be reasonably managed, abandoning responsibilities, engaging in promiscuous behavior, going on spending sprees, making numerous long distance phone calls, and so on. Such actions can be devastating to the patient and the patient's family and can adversely affect the patient's social and occupational functioning. Often, a patient can incur significant debt, health risks, and legal consequences. Untreated bipolar disorder can result in the patient's death and inadvertent harm to others. When manic patients are so impaired as to pose serious threats to their own safety or that of others, hospitalization is required.
If at least one manic episode has been experienced, the patient is said to have bipolar I disorder. There are syndromal variants in bipolar disorder, which differ in presentation, course, and treatment implications.3 Hypomania is shorter in duration and generally milder in intensity. Hence, while patients may appear noticeably giddy, happy, energetic, etc., they are not so impaired as to cause significant harm to themselves or others or lose contact with reality. In the course of bipolar disorder, patients usually experience profound periods of depression alternating with manic or hypomanic episodes. Patients who experience recurrent episodes of hypomania and depression, but no manic episodes, are said to have bipolar II disorder.2 In some cases, patients experience episodes during which criteria are simultaneously met for both depression and mania; they are said to have mixed mania.
The course of bipolar disorder is characterized by a high rate of recurrences.3,4 Patients vary in terms of the number of cycles that they may experience. Often, a cycle can be precipitated by sleep disturbances, life stresses, noncompliance with mood-stabilizing medications, or alcohol and/or illicit substance abuse. For a group of patients, cycles may occur as frequently as 4 or more times per year, which defines the rapid-cycling variant of bipolar disorder.2
Bipolar disorder affects approximately 1% of the population, or nearly 2 million persons.5 In 1991, bipolar disorder incurred $45 billion in costs: $7 billion for direct patient care, e.g., hospitalizations, medication use, and $38 billion for indirect costs, e.g., loss of wages, loss of work productivity, absenteeism.6
The consequences of bipolar disorder can be severe, enduring, and pervasive.7 Hence, efforts have long been directed at developing effective treatments for this disorder. Lithium carbonate was approved for treatment of mania in 1970 by the U.S. Food and Drug Administration (FDA). Until recently, lithium was considered the mainstay of treatment. However, severe adverse effects, potentially dangerous long-term consequences, and a narrow therapeutic index of safety limit the use of lithium.4,8,9 Furthermore, there is a subgroup of patients who demonstrate a poor response to lithium treatment.10,11 The effectiveness of lithium in acute mania and long-term maintenance treatment has been questioned by data on patient refractory rates and relapse rates. It is estimated that only 60% to 80% of patients with bipolar disorder have an adequate response to lithium treatment.12,13 Among patients with acute mania, lithium appears to be efficacious for patients with “classic” mania. Additionally, lithium is effective for patients with a cycle pattern of mania followed by depression and then euthymia. As many as 30% to 50% of bipolar patients, however, have a cycle pattern of depression followed by mania and then a euthymic period14,15; such patients tend to have a poorer response to lithium treatment. Patients with mixed mania,16–18 rapid cycling,19 and/or lacking a familial history of affective disorders15 are also less likely to respond to lithium.
Relapse rates are high despite compliance with lithium treatment and appear to increase over time. Estimated relapse rates are approximately 40% in the first year posttreatment20 and 73% in 5 years.21
The observations above propelled efforts to develop alternate treatment strategies for bipolar affective illness. The link between anticonvulsants and bipolar disorder emerged, in part, from observations that patients treated with anticonvulsants in early epilepsy trials experienced improvements in mood and general improvements in well-being.22–30 However, these studies were uncontrolled. The observed mood improvements may have been an artifact of better seizure control and commensurate lifestyle improvements and were not necessarily due to direct anticonvulsant effects. Nonetheless, these findings prompted some of the initial investigations into the possible effects of anticonvulsant use in psychiatric circles.
ANTICONVULSANT EFFICACY IN BIPOLAR DISORDER
Acute Mania
Patients refractory or intolerant to lithium are candidates for treatment with valproate (Table 1). Valproate received FDA approval for the treatment of acute mania in 1995. It is the only anticonvulsant thus far to receive FDA approval for treatment of mania. In 2 well-conducted, placebo-controlled, double-blind studies, valproate has been demonstrated to be more effective than placebo in acute mania.31,32 No significant differences were found in effectiveness between lithium-treated and valproate-treated groups when the latter was compared with a matched lithium-treated group.31 Subject dropout rates were higher for the lithium-treated group compared with the valproate-treated group, suggesting that valproate was better tolerated.31
Table 1.
Treatment Guidelines For Managing Bipolar Disorder
Alternatively, carbamazepine has demonstrated some promise in the treatment of acute mania.33 Acutely manic patients treated with carbamazepine demonstrated significant improvements compared with placebo controls; however, lower response rates were obtained with carbamazepine than with lithium.34,35
Preliminary evidence suggests that lamotrigine may also have a role in acute mania. The effectiveness of lamotrigine as an adjunctive agent in acute mania was suggested by case reports and a small open-label study (N = 36).36–38 In the open study, 60% of patients demonstrated a significant reduction in manic symptom severity as quantified on standardized psychiatric rating scales.38
Inconsistent, although promising, results were obtained in uncontrolled case reports,39,40 retrospective chart reviews,41,42 and open case series43,44 examining gabapentin adjunctive therapy in acute mania. One open-label study found that gabapentin (dose range, 1600–4800 mg/day) added to the preexisting treatment of 8 (88.9%) of 9 acutely manic patients resulted in moderate-to-marked improvements.45 There have been no trials of gabapentin monotherapy in acute mania.
The potential role of lamotrigine and/or gabapentin in acute mania will require prospective, controlled, double-blind testing. Furthermore, there have been no comparisons of the effectiveness of any of the anticonvulsants in acute mania published to date. Hence, statements of the efficacy of one agent over another cannot be made at this time. If there is poor response to valproate, combination treatment, i.e., carbamazepine or lithium along with valproate, should be attempted.4,46
Mixed Mania
Valproate is considered a first-line treatment for mixed mania. If ineffective, alternative treatments can include lithium, carbamazepine, or combinations of lithium and valproate.4,46 No studies examining the potential antimanic effects of gabapentin or lamotrigine have been conducted in mixed manic states as yet. As can be seen in Table 1, patients whose manic symptoms fail to respond to lithium or valproate may be treated with the alternate medication (valproate in the case of lithium nonresponse or vice versa), or supplemental agents. Partial responses to lithium or valproate may be best managed by adjunctive treatments, such as with lithium or carbamazepine. Combination therapies are common in the treatment of refractory bipolar disorder. Combinations of lithium and valproate tend to be safe and effective.47
Bipolar Depression
Patients may report that the experience of hypomania, and at times, mania, is pleasant. They may not have sufficient insight into the real and potential hazards that arise from behaviors emanating during such episodes. On the other hand, the experience of depressive episodes can be quite overwhelming. If ineffectively treated by lithium or alternative agents, patients may elect to discontinue their mood-stabilizing medications. In part, this decision may be driven by the assumption that such medications are inefficacious; for some patients, noncompliance is known to lead to recurrence of mania, which is perceived as a more pleasant emotional state. Hence, in addition to reducing the potential morbidity associated with mania, treatment must also reduce the pain and potential serious sequelae associated with depression.
There are no controlled studies assessing the efficacy of valproate in acute depression. However, valproate was relatively ineffective in the treatment of unipolar and bipolar depression in 4 open-label studies.48 When combined, the 4 studies demonstrated that only 58 (30%) of 195 patients responded to valproate use.
Carbamazepine, on the other hand, has been rather effective in treating bipolar depression. The plausibility of carbamazepine-associated antidepressant effects is reasonable considering that carbamazepine possesses a structure similar to that of tricyclic antidepressants.49 Longitudinal studies, employing an on-off-on design, demonstrated that carbamazepine was effective in reducing the severity of depressive episodes.50,51 Unfortunately, the sample sizes of the studies limit the generalizability of the results. In some cases, patients did not relapse into depression during the “off” period, i.e., with drug discontinuation, suggesting that mood improvement could not be unequivocally attributed to carbamazepine.51 An open study also supported that antidepressant efficacy in 17 (63%) of 27 carbamazepine-treated patients who entered remission after treatment. Controlled studies involving carbamazepine are lacking.
Lamotrigine, either as monotherapy or as adjunctive treatment, appears to have antidepressant effects. Case reports and 4 open-label studies suggest the benefit of supplemental lamotrigine in bipolar patients unresponsive or partially responsive to conventional treatment.36–38,52–55
A recent multicenter, randomized, double-blind study employing lamotrigine monotherapy in bipolar depression is worth noting.56 Patients (N = 192) with bipolar depression of at least 8 weeks' duration were randomly assigned to 1 of 3 conditions for a 7-week period: placebo; lamotrigine, 50 mg/day; or lamotrigine, 200 mg/day. Significant reductions in depressive symptoms were noted among lamotrigine-treated patients compared with the placebo-treated patients. Furthermore, a dose-response relationship was suggested by the clinical improvement of patients in the 200-mg/day group compared with those in the 50-mg/day group.
Among patients with bipolar depression treated with adjunctive gabapentin (mean ± SD dose = 1050 ± 640 mg/day) during a 6-week, open-label trial, 53% of patients (8/15) demonstrated a significant, albeit modest, improvement in scores on rating scales assessing depression severity.57 Another double-blind study employing gabapentin monotherapy in a small sample of bipolar patients revealed that antidepressant effects of gabapentin occurred within the first week of the trial, but rapidly dissipated by the fifth week of treatment.58
Of note, lamotrigine reportedly caused depressed patients to develop symptoms of hypomania.56 However, the rate of induced hypomania did not statistically significantly differ for lamotrigine-treated and placebo-treated patients. This suggests that the hypomania observed may have been part of the natural course of the bipolar affective illness for these patients, rather than an effect of the lamotrigine per se. By contrast, gabapentin was associated with development of hypomania and accelerated cycling in depressed patients.41,42,44,57,59,60 Caution, therefore, is required if trials of gabapentin are employed in depressed bipolar patients.
In general, when faced with bipolar depression refractory or intolerant to lithium, carbamazepine is a good alternative4,46 (see Table 1). While further research is required into its efficacy, lamotrigine may also be of benefit to depressed patients, either as an adjunct to existing treatment or as monotherapy. Gabapentin also requires further investigation; its use would have to be closely monitored to avoid precipitation of a manic episode. Data on the utility of gabapentin and lamotrigine were not available at the time treatment guidelines for bipolar disorder4,46 were published. Both agents were included in Table 1 since they have potential for treatment, particularly in bipolar depression.
Rapid Cycling
Both valproate and carbamazepine demonstrate efficacy in the treatment of patients with rapid-cycling bipolar disorders.31,61–63 Consequently, valproate and carbamazepine constitute the first- and second-line treatment recommendations for management of patients with rapid cycling.4,46 The effectiveness of lamotrigine and gabapentin in rapid cycling has yet to be studied in controlled conditions. Preliminary data from case reports and uncontrolled, small, open-label studies suggest lamotrigine's effectiveness among patients with rapid cycling.36,38,52,54,55,64 A double-blind, crossover trial of gabapentin and lamotrigine monotherapy suggested that patients with rapid cycling responded well to lamotrigine and, to a lesser extent, gabapentin monotherapy.58 The small sample sizes in these studies limit the generalizability of results obtained with lamotrigine. Patients unresponsive to valproate or carbamazepine monotherapy may benefit from combinations of those treatments with lithium4,46 (see Table 1).
Prophylaxis of Recurrent Episodes
In addition to the goal of reducing distress associated with acute episodes, pharmacologic interventions are also aimed at preventing future episodes for patients with bipolar disorder. Open-label studies suggest that valproate reduces the frequency and severity of mood episode recurrences in patients with rapid cycling, mixed bipolar, and bipolar II disorders.48,62,65–67 As noted previously, valproate may be more effective in reducing the frequency of manic episodes as opposed to depressive episodes.48,68–71 During a 2-year follow-up period, the mean number of episodes of mood disturbances for valproate-treated patients was less than, although not statistically significantly different from, that for lithium-treated patients.71
Preliminary data from a large double-blind study comparing placebo, lithium, and valproate prophylaxis over a 1-year span revealed that valproate-treated patients had fewer affective episodes (either manic or depressive) than either placebo- or lithium-treated patients.72
Data from several reports indicate that carbamazepine may effectively reduce the frequency and severity of recurrent episodes of bipolar disorder.35,73–81 Some of these studies involved comparisons of carbamazepine with placebo76 and lithium.35,73,75,77,81 While carbamazepine was found to be more effective in the prophylaxis of recurrences than placebo,76 comparisons with lithium were more difficult to interpret. Overall, no significant differences in efficacy were found between lithium and carbamazepine.35,73,75,77 Carbamazepine appeared to lose efficacy on long-term follow-up; in other words, relapse rates tended to be higher for carbamazepine-treated patients.35,73,77
A recent randomized multicenter comparison of the prophylactic effects of carbamazepine and lithium was conducted in 144 patients with bipolar disorder for a 2.5-year period. Several outcome measures were employed, including recurrence rates, need for hospitalization, need for concomitant medications, and reported rates of adverse effects. While the hospitalization rates for subjects in the 2 groups were comparable, recurrence rates and the rates of required supplemental psychotropics favored the lithium-treated group. In addition, when compared with lithium-treated subjects, significantly more carbamazepine-treated subjects reported adverse effects. In fact, treatment discontinuation due to side effects was higher among the carbamazepine-treated subjects than among lithium-treated subjects. Lithium may be more effective than carbamazepine in prophylaxis of recurrences of bipolar affective disorder and may be better tolerated for the long-term treatment of bipolar disorder.82
The prophylactic effects of lamotrigine and gabapentin as monotherapy or adjunctive mood-stabilizing agents have not been assessed as yet.
Consequently, lithium continues to be recommended as the first choice for prophylaxis.4,46 However, valproate would certainly constitute a reasonable alternative. Again, for patients with a history of rapid cycling, valproate may be a better option.
ISSUES IN ANTICONVULSANT USE
Primary care physicians caring for a patient with bipolar disorder will need to maintain close contact with the psychiatrist managing medications for bipolar disorder. The primary care physician will need to be aware of the safety and toxicity issues, side effect profiles, and drug interactions associated with anticonvulsant use. These will be described briefly below.
Valproate
Valproate is available in 4 formulations from Abbott Laboratories: valproic acid, divalproex sodium, divalproex sprinkle capsules, and sodium valproate syrup. Valproate is generally begun at 750 mg/day in divided doses. The dose is increased every 2 to 3 days as tolerated to a maximum dose of 1000 to 2000 mg/day. Valproate should be dosed to clinical responsiveness as well as patient tolerance of side effects. Although no definite therapeutic serum level has been established for the use of valproate, levels of more than 50 µg/mL may be associated with a better response in acute mania.67 The target ranges appear to be 50 to 125 µg/mL.
For hospitalized patients, oral loading of valproate may be particularly worthwhile. Doses are initiated at 20 mg/kg/day and increased as tolerated for clinical responsiveness. Doses are not to exceed 60 mg/kg/day. Oral loading of valproate is safe, rapidly achieves symptom remission,83 and reduces hospital length of stay.84,85 Oral loading may not be possible in the elderly and those with comorbid medical conditions, e.g., severe hepatic disease.
The side effects associated with valproate use are summarized in Table 2. Gastrointestinal disturbances are most common. Nausea may be reduced with the use of the sustained-release formulations, such as divalproex sodium.86,87 However, there is a greater risk of diarrhea with the sustained-release formulations. Tremor develops in approximately 10% of valproate-treated patients, but can be reduced with low-dose propranolol or amantadine.88 Valproate functions as a chelating agent, affecting zinc and selenium levels. Patients so affected may experience transient hair loss. Drug discontinuation is unnecessary, since patients can be treated with selenium and zinc supplements.89
Table 2.
Side Effects Associated With Anticonvulsants Used in Bipolar Disorder
Valproate use is also associated with the possibility of elevation of liver enzymes and thrombocytopenia. Transient elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) have been reported in as many as 11% of valproate-treated patients.90 However, increases in liver enzymes are generally asymptomatic and usually return to baseline with dose reduction or drug discontinuation. Rare cases of fatal hepatotoxicity have been reported. Similarly, thrombocytopenia has been reported (see Table 2). Thrombocytopenia and hepatotoxicity are rare occurrences early in treatment with valproate. The risk of fatal hepatotoxicity appears to be highest in very young patients, i.e., patients under age 2 years, and declines with age.91 Furthermore, rates of valproate-associated hepatic toxicity increases in patients receiving multiple anticonvulsant medications. Significantly reduced platelet counts or evidence of bleeding and excessive bruising during the course of valproate treatment would warrant drug discontinuation.
Valproate has also been implicated in producing variable and, at times, conflicting changes in thyroid indices92–95; however, these do not appear to be clinically significant. Very rarely, pancreatic inflammation has been reported with valproate use, usually in younger patients and generally early in the course of treatment.96 Patients should be informed to contact their physicians if any of the following symptoms occur: unusual bleeding and bruising, right upper quadrant abdominal pain/tenderness, jaundice, darkening of the urine, stools that appear pale, fever, severe confusion, double vision, or severe trembling. The latter 3 symptoms usually signal valproate toxicity and warrant evaluation of serum valproate levels.
The half-life of valproate is 9 to 16 hours. Monitoring serum valproate concentrations is recommended approximately 2 to 3 days after drug initiation or dose increase. The serum level should be obtained in the morning, before the first scheduled dose of the day, to avoid obtaining falsely elevated serum levels. Ideally, to avoid toxicity, serum valproate levels should not exceed 125 µg/mL. In addition to assessing for toxicity, monitoring of serum levels may be helpful when drug-drug interactions that increase valproate levels are suspected or to determine patient compliance. Otherwise, it has been argued that measuring plasma concentrations is of little value if patients are otherwise clinically well controlled.
Because the risk of hepatotoxicity is highest early in the course of treatment, liver function tests should be conducted at monthly intervals during the first 6 months of treatment, and less frequently thereafter.97 Routine monitoring of pancreatic enzymes is generally not required unless prompted by symptoms of pancreatic inflammation, e.g., epigastric abdominal pain, vomiting, and anorexia. Similarly, routine thyroid function testing is generally not required.
Women of reproductive age should be asked if there are any pregnancies planned, and if so, alternatives to valproate should be explored. There is an increased risk of neural tube defects98–101 and midline organ malformations102,103 associated with valproate use, particularly in the first trimester (Table 3). Valproate is also secreted in breast milk during lactation, but its effects on the developing infant are unknown.
Table 3.
Safety of Anticonvulsants Used in Bipolar Disorder in Pregnancy and Lactationa
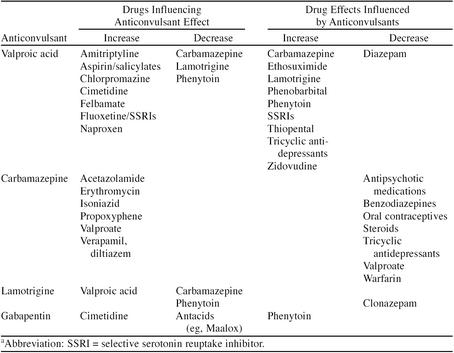
Pertinent drug interactions associated with valproate use are listed in Table 4. Valproate tends to inhibit drug oxidation, thereby increasing concentrations of a number of potentially coadministered medications. Medications that displace valproate from protein binding, such as salicylates, can increase valproate levels. Similarly, agents that enhance drug metabolism, such as carbamazepine, can reduce valproate levels.
Table 4.
Drug Interactions Associated With Anticonvulsants Used in Bipolar Disordera
Carbamazepine
Carbamazepine is employed in doses of 10 to 20 mg/kg/day. The initial dose may need to be lower, e.g., 3 to 5 mg/kg/day, and increased slowly every 5 to 7 days until the desired clinical effect is achieved or until limited by side effects. This approach will determine the lowest effective dose or maximal tolerated dose.
The dose can be administered in conventional tablets or liquid suspension. The total daily dose should be administered in 3 to 4 divided doses to avoid large fluctuations in serum levels. However, in cases where repeated doses produce sedation or interfere with compliance, the doses can be administered twice a day. Slow-release preparations can be used as well.
The side effects associated with carbamazepine use are summarized in Table 2. Neurologic symptoms, such as diplopia, nystagmus, confusion, and ataxia, are dose related and remit with dose reductions. Less common untoward effects associated with carbamazepine use include elevations in the liver enzymes (5%–15% of patients), hyponatremia (6%–31%), and rash (10%–12%).49 Leukopenia usually occurs early in the course of treatment. Often the white blood cell counts return to baseline without carbamazepine dose modifications.104–106 Rarely, severe leukopenia, i.e., white blood cell counts below 3000/mm3 or neutrophils below 1500/mm3, has occurred, requiring drug discontinuation.107 Few cases of thrombocytopenia108 and rare cases of aplastic anemia104 were reported.
Carbamazepine-associated rash can be treated with steroids. It rarely leads to bleeding or extensive exfoliation, such as in Stevens-Johnson syndrome. Severe hyponatremia has been associated with carbamazepine use, associated with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH).109,110 The mechanisms for carbamazepine-associated SIADH are unclear. Patients may experience severe headache, fatigue, vertigo, and confusion associated with the hyponatremia. Remediation may arise with fluid restriction, carbamazepine discontinuation, and, if necessary, demeclocycline administration.109,111 Hyponatremia is apt to occur in adults, especially elderly patients. Patients should be apprised of the need to notify their physicians if dermatologic, hepatic, infectious, or hematologic (i.e., bruising and bleeding) symptoms develop. Routine follow-up with patients is required. Routine laboratory examinations may be helpful, but cannot anticipate whether potentially fatal idiosyncratic reactions will occur.
Carbamazepine is implicated in producing reductions in free thyroxine and total thyroxine serum levels as well as serum triiodothyronine levels.92,93,95 However, clinically significant hypothyroidism has only rarely been reported.112
Unlike the therapeutic serum levels found with lithium and valproate, there does not appear to be such a serum level of carbamazepine for acute mania. Previous researchers have reported that acutely manic patients who responded to carbamazepine had serum levels between 4 and 15 µg/mL.50,113
Because leukopenia tends to occur early in the course of treatment, periodic monitoring of complete blood counts should be undertaken. Granulocyte counts are warranted in patients presenting with fever and signs of infection. Routine monitoring of liver enzymes is generally not required unless prompted by signs or symptoms of hepatic inflammation, e.g., right upper quadrant abdominal pain, vomiting, anorexia, jaundice, darkened urine, pale colored stools. Routine thyroid function testing is generally not required.
Monitoring of serum drug concentrations can be helpful in evaluating patient compliance or concentration-dependent toxicity. Because of the half-life of carbamazepine, serum carbamazepine concentrations take 3 to 7 days to stabilize. Serum levels should be obtained in the morning, prior to the first daily drug administration for that day.
It is important to remember that carbamazepine activates the hepatic cytochrome P450 enzyme system, inducing those same enzymes that are responsible for its metabolism. Consequently, serum carbamazepine levels may dramatically decrease after a period of time. Hence, the dosage of the carbamazepine taken daily may need to be increased to return to previously effective serum levels at which the patient had been doing well. Such autoinduction of the P450 isoenzymes is likely to occur early in treatment.114
Carbamazepine treatment during pregnancy is associated with a number of potential fetal malformations,115–117 as can be seen in Table 3. The risk of neural tube defects may be reduced during pregnancy with folic acid supplementation in doses of 1 to 4 mg/day in the 4 weeks prior to conception if the pregnancy is planned and throughout the first trimester.103,118 Carbamazepine is also secreted in breast milk,119–121 but the long-term effects on the newborn are unknown. Breastfeeding should not be discouraged unless the newborn demonstrates side effects or signs of toxicity associated with carbamazepine.122
Multiple drug interactions are possible with carbamazepine123,124; several of these are summarized in Table 4. Women, particularly those who wish to avoid pregnancy during carbamazepine treatment, must be apprised of the increased risk of oral contraceptive failures attributed to concomitant carbamazepine use.103,113 Additionally, while not listed in the table, combinations of carbamazepine with other mood-stabilizing agents may be associated with the possibility of enhanced neurotoxicity.125 There may be an increased risk of cognitive deficits associated with combinations of carbamazepine and lithium.126 The risk of agranulocytosis may be increased if carbamazepine is coadministered with medications potentially producing comparable effects, such as clozapine.
Lamotrigine
Lamotrigine is initiated at 25 mg/day and increased by an additional 25 mg every 2 weeks until the target dose, i.e., 200 to 500 mg/day, is achieved or until side effects preclude further dose increases. Due to the risk of dermatologic side effects, such as rash, the manufacturer cautions against more rapid dose escalations. If the patient is concurrently treated with valproate, the aforementioned dosing regimen is halved.38 Dose reductions (and corresponding slower dosing increments) are warranted in patients with significant hepatic or renal impairments.
Common adverse reactions associated with lamotrigine are summarized in Table 2. A macular-papular or erythematous rash developed in approximately 10% of 3501 individuals receiving lamotrigine in epilepsy trials.127–129 Drug discontinuation due to the rash was warranted only in 3.8% of patients, while the rash resolved in the remaining patients despite continued lamotrigine treatment.127–129 For unknown reasons, the incidence of rash and subsequent drug discontinuation due to the rash has been higher in trials of patients with bipolar disorder.38,56 Life-threatening rashes, such as Stevens-Johnson syndrome or toxic epidermal necrolysis, occur in 1/1000 treated adults. Patients should contact their physicians if a rash develops during lamotrigine treatment. Rash occurrence is highest in the first 4 to 6 weeks of lamotrigine treatment. In addition, children appear to be particularly vulnerable to rash development; the risk of developing a life-threatening rash is approximated to be 1/50 to 1/100. Lamotrigine should be avoided in young patients, aged 16 years or less. Other factors associated with rash development include coadministration of lamotrigine and valproate, exceeding the recommended initial dose of lamotrigine, or overly aggressive lamotrigine dose escalation.
Animal studies reveal few teratogenic effects.102 Safe use in pregnancy has not yet been established, but may offer some advantages over valproate and carbamazepine.130 Because lamotrigine passes into breast milk, nursing while treated with lamotrigine is not recommended (Table 3).
There are a limited number of side effects associated with lamotrigine use, summarized in Table 4. Coadministration of lamotrigine with valproate is associated with an increased risk of life-threatening rash.
Gabapentin
Gabapentin is dosed flexibly with a starting dose of 300 mg b.i.d. The dose can be increased gradually up to a maximal dose of 3600 mg/day. It should be administered in divided doses due to its short half-life (5–9 hours) to maintain adequate serum levels.131
Gabapentin has no known potentially fatal effects and few side effects (Table 2). Gabapentin is excreted unchanged from the kidneys; plasma clearance is proportional to the creatinine clearance.131,132 Dose reductions are required for patients who have compromised renal functioning or require dialysis.133–135 Fetal risks associated with gabapentin use in pregnancy and lactation are as yet unknown (Table 4), nor has the safety of use in children under the age of 12 years been established.
Gabapentin circulates unbound to plasma proteins and is not appreciably metabolized.136 Hence, there are no problems arising from coadministration with highly protein-bound medications and virtually no effect on the hepatic cytochrome P450 system, and therefore, there are minimal drug interactions associated with gabapentin131,132,137 (Table 3). Possible drug interactions between phenytoin and gabapentin have been reported.138,139
OTHER ANTICONVULSANTS WITH POTENTIAL FOR USE IN BIPOLAR DISORDER
Increasing attention may be directed at topiramate and other relatively new anticonvulsants, vigabatrin and tigabine, for use in bipolar disorder. The efficacy and safety of these agents in bipolar patients are yet unknown. A preliminary study of the effect of topiramate in 11 acutely manic patients (dose range, 50–1300 mg/day) demonstrated moderate-to-marked improvements in 5 patients (45%).140 Further study is required to assess the utility of these medications in bipolar patients.
SUMMARY
While lithium carbonate had been the mainstay of treatment, there is increasing evidence for the role of anticonvulsants in the treatment of syndromal variants of bipolar disorder. Valproate is the only anticonvulsant thus far approved by the FDA for treatment of acute mania. It is considered the first-line treatment for mixed mania and rapid cycling, as well as a good alternative for lithium failure or intolerance in the treatment of mania. It appears to be efficacious in the long-term maintenance treatment of bipolar disorder, although not as yet officially FDA approved for this indication. Carbamazepine is a good alternative to valproate in the treatment of rapid cycling and to lithium in the treatment of bipolar depression. Both valproate and carbamazepine are associated with a number of adverse effects and potential drug interactions that necessitate careful patient follow-up and periodic laboratory investigations.
The roles of lamotrigine and gabapentin in the treatment of bipolar disorder (either as monotherapy or as supplemental agents) have as yet to be clarified. Lamotrigine may be of particular benefit in bipolar depression. It is associated with few drug interactions and may be safer than valproate or carbamazepine in pregnancy. The data, thus far, on the efficacy of gabapentin in bipolar disorder are limited, but warrant further investigation. Gabapentin is safe in patients with significant hepatic disease or who sustain hepatic complications associated with use of valproate or carbamazepine. The lack of significant drug interactions make it safe in patients requiring multiple psychotropic medications. It has virtually no impact on protein-bound medications and does not effect the cytochrome P450 system.
Despite the advances in pharmacologic management of bipolar disorder, it remains a chronic disease with frequent recurrences and exacerbations. Uncontrolled, this disorder can have profound and enduring effects. Pharmacologic treatments that can offset phases of the illness or the recurrent cycles are desperately needed. Future research will assess the utility and safety associated with newer anticonvulsants as well. Familiarity with the expanding treatment options available in bipolar disorder will render the primary care physician effective, working alone or in collaboration with a psychiatrist, in addressing management issues, adverse medication effects, and the potential for drug interactions.
Drug names: amantadine (Symmetrel and others), amitriptyline (Elavil and others), carbamazepine (Tegretol and others), chlorpromazine (Thorazine and others), cimetidine (Tagamet and others), clonazepam (Klonopin and others), clozapine (Clozaril), demeclocyline (Declomycin), diazepam (Valium and others), diltiazem (Cardizem and others), divalproex sodium (Depakote), ethosuximide (Zarontin), felbamate (Felbatol), fluoxetine (Prozac), gabapentin (Neurontin), isoniazid (Rifamate and others), lamotrigine (Lamictal), lithium (Eskalith and others), naproxen (Naprosyn and others), phenytoin (Dilantin and others), propoxyphene (Darvocet and others), propranolol (Inderal and others), topiramate (Topamax), valproic acid (Depakene), verapamil (Calan and others), warfarin (Coumadin), zidovudine (Retrovir).
Acknowledgments
The authors thank Constance Sherry, R.N., M.S., for suggestions in the preparation of this manuscript.
REFERENCES
- Manning JS, Haykal RF, Connor PD, et al. On the nature of depressive and anxious states in a family practice setting: the high prevalence of bipolar II and related disorders in a cohort followed longitudinally. Compr Psychiatry. 1997;38:102–108. doi: 10.1016/s0010-440x(97)90089-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness. New York, NY: Oxford University Press. 1990 [Google Scholar]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients With Bipolar Disorder. Am J Psychiatry. 1994;151(12, suppl):1–36. doi: 10.1176/ajp.151.12.1. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1985;18:141–153. doi: 10.1017/s0033291700001975. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness, 1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30:213–219. doi: 10.1007/BF00789056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M, et al. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150:720–727. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Kane JM, Keller MB, et al. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorders. N Engl J Med. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- Lenox RH, Manji HK. Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. Washington, DC: American Psychiatric Press. 1998 379–430. [Google Scholar]
- Post RM. Alternatives to lithium for bipolar affective illness. In: Tasman A, Goldfinger SM, Kaufman CA, eds. Review of Psychiatry, vol 9. Washington, DC: American Psychiatric Press. 1990 170–202. [Google Scholar]
- Post RM. Prophylaxis of bipolar affective disorders. Int Rev Psychiatry. 1990;2:277–320. [Google Scholar]
- Calabrese JR, Fatemi SH, Kujawa M, and et al. Predictors of response to mood stabilizers. J Clin Psychopharmacol. 1996 16(suppl). 24–31. [DOI] [PubMed] [Google Scholar]
- Harrow M, Goldberg JF, Grossman LS, et al. Outcome in manic disorders: a naturalistic follow-up study. Arch Gen Psychiatry. 1990;47:665–671. doi: 10.1001/archpsyc.1990.01810190065009. [DOI] [PubMed] [Google Scholar]
- Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic-depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156–167. doi: 10.1055/s-2007-1019628. [DOI] [PubMed] [Google Scholar]
- Maj M, Pirozzi R, Starace F. Previous pattern of course of the illness as a predictor of response to lithium prophylaxis in bipolar patients. J Affect Disord. 1989;17:237–241. doi: 10.1016/0165-0327(89)90005-0. [DOI] [PubMed] [Google Scholar]
- Bowden CL. Predictors of response to divalproex and lithium. J Clin Psychiatry. 1995;56(suppl 3):25–30. [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Coryell W, et al. Differential outcome of pure manic, mixed/cycling, and pure depressive episodes in patients with bipolar illness. JAMA. 1986;255:3138–3142. [PubMed] [Google Scholar]
- Swann AC, Bowden CL, Morris D, et al. Depression during mania: treatment response to lithium or divalproex. Arch Gen Psychiatry. 1997;54:37–42. doi: 10.1001/archpsyc.1997.01830130041008. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Woyshville MJ, Kimmel SE, et al. Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacol. 1993;13:280–283. [PubMed] [Google Scholar]
- O'Connell RA, Mayo JA, Flatow L, et al. Outcome in bipolar disorder on long-term treatment with lithium. Br J Psychiatry. 1991;159:123–129. doi: 10.1192/bjp.159.1.123. [DOI] [PubMed] [Google Scholar]
- Gitlin MJ, Swendsen J, Heller TL, et al. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152:1635–1640. doi: 10.1176/ajp.152.11.1635. [DOI] [PubMed] [Google Scholar]
- Dalby MA. Behavioral effects of carbamazepine. In: Penry J, Daly DD, eds. Advances in Neurology, II. New York, NY: Raven Press. 1975 331–344. [PubMed] [Google Scholar]
- Diamond KR, Pande AC, Lamoreaux L, et al. Effect of gabapentin on mood and well-being in patients with epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:407–417. doi: 10.1016/0278-5846(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Handforth A, Treiman DM. Efficacy and tolerance of long-term, high dose gabapentin: additional observations. Epilepsia. 1994;35:1032–1037. doi: 10.1111/j.1528-1157.1994.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Harden C, Pick L. Alterations in mood and anxiety in epilepsy patients treated with gabapentin [abstract]. Epilepsia. 1996 37(suppl). 137. [DOI] [PubMed] [Google Scholar]
- Ojemann LM, Wilensky AJ, Temkin NR, et al. Long-term treatment with gabapentin for partial epilepsy. Epilepsy Res. 1992;13:159–165. doi: 10.1016/0920-1211(92)90072-2. [DOI] [PubMed] [Google Scholar]
- Smith D, Baker G, Davies G, et al. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34:312–322. doi: 10.1111/j.1528-1157.1993.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Smith D, Chadwick D, Baker G, et al. Seizure severity and the quality of life. Epilepsia. 1993;34(suppl 5):S31–S35. doi: 10.1111/j.1528-1157.1993.tb05921.x. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Trimble MR. Anticonvulsant drugs and cognitive functions. Epilepsia. 1982;23:531–545. doi: 10.1111/j.1528-1157.1982.tb05439.x. [DOI] [PubMed] [Google Scholar]
- Trimble MR. Carbamazepine and mood: evidence from patients with seizure disorders. J Clin Psychiatry. 1988;49(4, suppl):7–11. [PubMed] [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. JAMA. 1994;271:918–924. [PubMed] [Google Scholar]
- Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. Arch Gen Psychiatry. 1991;48:62–68. doi: 10.1001/archpsyc.1991.01810250064008. [DOI] [PubMed] [Google Scholar]
- Post RM, Ketter TA, Denicoff K, et al. The place of anticonvulsant therapy in bipolar illness. Psychopharmacology (Berl) 1996;128:115–129. doi: 10.1007/s002130050117. [DOI] [PubMed] [Google Scholar]
- Lerer B, Moore N, Meyendorff E, et al. Carbamazepine versus lithium in mania: a double-blind study. J Clin Psychiatry. 1987;48:89–93. [PubMed] [Google Scholar]
- Small JG, Klapper MH, Milstein V, et al. Carbamazepine compared with lithium in the treatment of mania. Arch Gen Psychiatry. 1991;48:915–921. doi: 10.1001/archpsyc.1991.01810340047006. [DOI] [PubMed] [Google Scholar]
- Fogelson DL, Sternbach H. Lamotrigine treatment of refractory bipolar disorder [letter] J Clin Psychiatry. 1997;58:271–273. doi: 10.4088/jcp.v58n0607b. [DOI] [PubMed] [Google Scholar]
- Walden J, Hesslinger B, van Calker D, et al. Addition of lamotrigine to valproate may enhance efficacy in the treatment of bipolar affective disorder. Pharmacopsychiatry. 1996;29:193–195. doi: 10.1055/s-2007-979570. [DOI] [PubMed] [Google Scholar]
- Corn T, Ascher J, Calabrese JR, and et al. Lamictal in the treatment of bipolar disorder [poster]. Presented at the 35th annual meeting of the American College of Neuropsychopharmacology. 9–13December1996 San Juan, Puerto Rico. [Google Scholar]
- Ryback R, Ryback L. Gabapentin for behavioral dyscontrol [letter] Am J Psychiatry. 1995;152:1399. doi: 10.1176/ajp.152.9.1399a. [DOI] [PubMed] [Google Scholar]
- Stanton SP, Keck PE Jr, McElroy SL. Treatment of acute mania with gabapentin [letter] Am J Psychiatry. 1997;154:287. doi: 10.1176/ajp.154.2.287a. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Katzow JJ, Desai SP, et al. Gabapentin treatment of mood disorders: a preliminary study. J Clin Psychiatry. 1998;59:426–429. doi: 10.4088/jcp.v59n0805. [DOI] [PubMed] [Google Scholar]
- Ryback RS, Brodsky L, Munasifi F. Gabapentin in bipolar disorder [letter] J Neuropsychiatry. 1997;9:301. doi: 10.1176/jnp.9.2.301b. [DOI] [PubMed] [Google Scholar]
- Bennett J, Goldman WT, Suppes T. Gabapentin for treatment of bipolar disorder and schizoaffective disorders. J Clin Psychopharmacol. 1997;17:141–142. doi: 10.1097/00004714-199704000-00029. [DOI] [PubMed] [Google Scholar]
- Schaffer CB, Schaffer LC. Gabapentin in the treatment of bipolar disorder [letter] Am J Psychiatry. 1997;154:291–292. doi: 10.1176/ajp.154.2.291. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Soutullo CA, Keck PE, et al. A pilot trial of adjunctive gabapentin in the treatment of bipolar disorder. Ann Clin Psychiatry. 1997;9:99–103. doi: 10.1023/a:1026257303275. [DOI] [PubMed] [Google Scholar]
- Expert Consensus Guideline Series: Treatment of Bipolar Disorder. J Clin Psychiatry. 1996;57(suppl 12A):1–89. [PubMed] [Google Scholar]
- Freeman MP, Stoll AL. Mood stabilizer combinations: a review of safety and efficacy. Am J Psychiatry. 1998;155:12–21. doi: 10.1176/ajp.155.1.12. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Keck PE, Pope HG, et al. Valproate in the treatment of bipolar disorder: literature review and clinical guidelines. J Clin Psychopharmacol. 1992;12(1, suppl):42S–52S. doi: 10.1097/00004714-199202001-00007. [DOI] [PubMed] [Google Scholar]
- Keck PE, McElroy SL. Antiepileptic drugs. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. Washington, DC: American Psychiatric Press. 1998 431–454. [Google Scholar]
- Ballenger JC, Post RM. Carbamazepine in manic-depressive illness: a new treatment. Am J Psychiatry. 1980;137:782–790. doi: 10.1176/ajp.137.7.782. [DOI] [PubMed] [Google Scholar]
- Post RM, Uhde TW, Roy-Byrne PP, et al. Antidepressant effects of carbamazepine. Am J Psychiatry. 1986;143:29–34. doi: 10.1176/ajp.143.1.29. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58:522–527. doi: 10.4088/jcp.v58n1202. [DOI] [PubMed] [Google Scholar]
- Kotler M, Matar MA. Lamotrigine in the treatment of resistant bipolar disorder. Clin Neuropharmacol. 1998;21:65–67. [PubMed] [Google Scholar]
- Kusumakar V, Yatham L. An open study of lamotrigine in refractory bipolar depression. Psychiatry Res. 1997;72:145–148. doi: 10.1016/s0165-1781(97)00082-6. [DOI] [PubMed] [Google Scholar]
- Weisler R, Risner ME, Ascher J, and et al. Use of lamotrigine in the treatment of bipolar disorder [poster]. Presented at the 147th Annual Meeting of the American Psychiatric Association. 21–26May1994 Philadelphia, Pa. [Google Scholar]
- Bowden CL, Calabrese JR, Sachs GS, and et al. Lamotrigine in bipolar depression. In: New Research Program and Abstracts of the 151st Annual Meeting of the American Psychiatric Association. May 30–June 4, 1998 Toronto, Ontario, Canada. Abstract NR654:244. [Google Scholar]
- Young LT, Robb JC, Patelis-Siotis I, et al. Acute treatment of bipolar depression with gabapentin. Biol Psychiatry. 1997;42:851–853. doi: 10.1016/s0006-3223(97)00305-3. [DOI] [PubMed] [Google Scholar]
- Frye MA, Ketter TA, Osuch EA, and et al. Gabapentin and lamotrigine monotherapy in mood disorder: an update. In: Syllabus and Proceedings Summary of the 151st Annual Meeting of the American Psychiatric Association. May 30–June 4, 1998 Toronto, Ontario, Canada. No. 77D:150. [Google Scholar]
- Marcotte DB, Fogleman L, Wolfe N, and et al. Gabapentin: an effective therapy for patients with bipolar affective disorder. In: New Research Program and Abstracts of the 150th Annual Meeting of the American Psychiatric Association. 20May1997 San Diego, Calif. Abstract NR261:138. [Google Scholar]
- Short C, Cooke L. Hypomania induced by gabapentin [letter] Br J Psychiatry. 1995;166:679–680. doi: 10.1192/bjp.166.5.679b. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Delucchi GA. Phenomenology of rapid cycling manic depression and its treatment with valproate. J Clin Psychiatry. 1989;50(3, suppl):30–34. [PubMed] [Google Scholar]
- Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 patients with rapid-cycling bipolar disorder. Am J Psychiatry. 1990;147:431–434. doi: 10.1176/ajp.147.4.431. [DOI] [PubMed] [Google Scholar]
- Post RM, Uhde TW, Ballenger JC, et al. Prophylactic efficacy of carbamazepine in manic-depressive illness. Am J Psychiatry. 1983;140:1602–1604. doi: 10.1176/ajp.140.12.1602. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Fatemi SH, Woyshville MJ. Antidepressant effects of lamotrigine in rapid cycling bipolar disorder [letter] Am J Psychiatry. 1996;153:1236. doi: 10.1176/ajp.153.9.1236a. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Markovitz PJ, Kimmell SE, et al. Spectrum of efficacy of valproate in 78 rapid-cycling bipolar patients. J Clin Psychopharmacol. 1992;12(1, suppl):53S–56S. doi: 10.1097/00004714-199202001-00008. [DOI] [PubMed] [Google Scholar]
- Guscott R. Clinical experience with valproic acid in 22 patients with refractory bipolar mood disorder [letter] Can J Psychiatry. 1992;37:590. doi: 10.1177/070674379203700820. [DOI] [PubMed] [Google Scholar]
- Hayes SG. Long-term use of valproate in primary psychiatric disorders. J Clin Psychiatry. 1989;50(3, suppl):35–39. [PubMed] [Google Scholar]
- McElroy SL, Keck PE Jr, Pope HG Jr, et al. Valproate in psychiatric disorders: literature review and clinical guidelines. J Clin Psychiatry. 1989;50(3, suppl):23–29. [PubMed] [Google Scholar]
- Puzynski S, Klosiewicz L. Valproic acid amide in the treatment of affective and schizoaffective disorders. J Affect Disord. 1984;6:115–121. doi: 10.1016/0165-0327(84)90013-2. [DOI] [PubMed] [Google Scholar]
- Puzynski S, Klosiewicz L. Valproic acid amide as a prophylactic agent in affective and schizoaffective disorders. In: Emrich HM, Okuma T, Muller AA, eds. Anticonvulsants in Affective Disorders. Amsterdam, the Netherlands: Excerpta Medica. 1984 68–75. [PubMed] [Google Scholar]
- Puzynski S, Klosiewicz L. Valproic acid amide as a prophylactic agent in affective and schizoaffective disorders. Psychopharmacol Bull. 1984;20:151–159. [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, and et al. Maintenance treatment in bipolar disorder. In: Syllabus and Proceedings Summary of the 151st Annual Meeting of the American Psychiatric Association. May 30–June 4, 1998 Toronto, Ontario, Canada. No. 6C:278. [Google Scholar]
- Coxhead N, Silverstone T, Cookson J. Carbamazepine versus lithium in the prophylaxis of bipolar affective disorder. Acta Psychiatr Scand. 1992;85:114–118. doi: 10.1111/j.1600-0447.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto A, Ogura C, Hazama H, et al. Long-term prophylactic effects of carbamazepine in affective disorder. Br J Psychiatry. 1983;143:327–331. doi: 10.1192/bjp.143.4.327. [DOI] [PubMed] [Google Scholar]
- Lusznat RM, Murphy DP, Nunn CMH. Carbamazepine vs lithium in the treatment and prophylaxis of mania. Br J Psychiatry. 1988;153:198–204. doi: 10.1192/bjp.153.2.198. [DOI] [PubMed] [Google Scholar]
- Okuma T, Inanaga K, Otsuki S, et al. A preliminary double-blind study on the efficacy of carbamazepine in prophylaxis of manic-depressive illness. Psychopharmacology (Berl) 1981;73:95–96. doi: 10.1007/BF00431111. [DOI] [PubMed] [Google Scholar]
- Placidi GF, Lenzi A, Lazzerini F, et al. The comparative efficacy and safety of carbamazepine versus lithium: a randomized, double-blind 3-year trial in 83 patients. J Clin Psychiatry. 1986;47:490–494. [PubMed] [Google Scholar]
- Post RM, Leverich GS, Rosoff AS, et al. Carbamazepine prophylaxis in refractory affective disorders: a focus on long-term follow-up. J Clin Psychopharmacol. 1990;10:318–327. [PubMed] [Google Scholar]
- Post RM, Uhde TW, Ballenger JC, et al. Prophylactic efficacy of carbamazepine in manic-depressive illness. Am J Psychiatry. 1983;140:1602–1604. doi: 10.1176/ajp.140.12.1602. [DOI] [PubMed] [Google Scholar]
- Stuppaeck C, Barnas C, Miller C, et al. Carbamazepine in the prophylaxis of mood disorders. J Clin Psychopharmacol. 1990;10:39–42. doi: 10.1097/00004714-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Watkins SE, Callender K, Thomas DR, et al. The effect of carbamazepine and lithium on remission from affective illness. Br J Psychiatry. 1987;150:180–182. doi: 10.1192/bjp.150.2.180. [DOI] [PubMed] [Google Scholar]
- Greil W, Ludwig-Mayerhofer W, Erazo N, et al. Lithium versus carbamazepine in the maintenance treatment of bipolar disorders: a randomised study. J Affect Disord. 1997;43:151–161. doi: 10.1016/s0165-0327(96)01427-9. [DOI] [PubMed] [Google Scholar]
- Keck PE Jr, McElroy SL, Tugrul KC, et al. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry. 1993;54:305–308. [PubMed] [Google Scholar]
- Keck PE Jr, McElroy SL, Bennett JA. Health-economic implications of the onset of action of antimanic agents. J Clin Psychiatry. 1996;57(suppl 13):13–18. [PubMed] [Google Scholar]
- Keck PE Jr, Nabulsi AA, Taylor JL, et al. A pharmacoeconomic model of divalproex vs. lithium in the acute and prophylactic treatment of bipolar I disorder. J Clin Psychiatry. 1996;57:213–222. [PubMed] [Google Scholar]
- Wilder BJ, Karas BJ, Penry JK, et al. Gastrointestinal tolerance of divalproex sodium. Neurology. 1983;33:808–811. doi: 10.1212/wnl.33.6.808. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Tohen M, Narendran R, et al. The adverse effect profile and efficacy of divalproex sodium compared with valproic acid: a pharmacoepidemiology study. J Clin Psychiatry. 1999;60:232–236. doi: 10.4088/jcp.v60n0405. [DOI] [PubMed] [Google Scholar]
- Karas BJ, Wilder BJ, Hammond EJ, et al. Treatment of valproate tremors. Neurology. 1983;33:1380–1382. doi: 10.1212/wnl.33.10.1380. [DOI] [PubMed] [Google Scholar]
- Bowden CL. Dosing strategies and time course of response to antimanic drugs. J Clin Psychiatry. 1996;57(suppl 13):4–9. [PubMed] [Google Scholar]
- Balfour JA, Bryson HM. Valproic acid: a review of its pharmacology and therapeutic potential in indications other than epilepsy. CNS Drugs. 1994;2:144–173. [Google Scholar]
- Dreifuss FE, Santilli N, Langer DH, et al. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37:379–385. doi: 10.1212/wnl.37.3.379. [DOI] [PubMed] [Google Scholar]
- Connacher AA, Borsey DQ, Browning MCK, et al. The effective evaluation of thyroid status in patients on phenytoin, carbamazepine or sodium valproate attending an epilepsy clinic. Postgrad Med J. 1987;63:841–845. doi: 10.1136/pgmj.63.744.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isojarvi JIT, Pakarinen AJ, Myllyla VV. Thyroid function with antiepileptic drugs. Epilepsia. 1992;33:142–148. doi: 10.1111/j.1528-1157.1992.tb02297.x. [DOI] [PubMed] [Google Scholar]
- Larkin JG, Macphee GJA, Beastall GH, et al. Thyroid hormone concentrations in epileptic patients. Eur J Clin Pharmacol. 1989;36:213–216. doi: 10.1007/BF00558149. [DOI] [PubMed] [Google Scholar]
- Verma NP, Haidukewych D. Differential but infrequent alterations of hepatic enzyme levels and thyroid hormone levels by anticonvulsant drugs. Arch Neurol. 1994;51:381–383. doi: 10.1001/archneur.1994.00540160079010. [DOI] [PubMed] [Google Scholar]
- Asconape JJ, Penry JK, Dreifuss FE, et al. Valproate-associated pancreatitis. Epilepsia. 1993;34:177–183. doi: 10.1111/j.1528-1157.1993.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Konig SA, Siemes H, Blaker F, et al. Severe hepatotoxicity during valproate therapy: an update and report of eight new fatalities. Epilepsia. 1994;35:1005–1015. doi: 10.1111/j.1528-1157.1994.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Dansky LV, Rosenblatt DS, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42(4, suppl 5):32–42. [PubMed] [Google Scholar]
- Lindhout D, Omtzigt JGC, Cornel MC. Spectrum of neural-tube defects in 34 infants prenatally exposed to antiepileptic drugs. Neurology. 1992;42(4, suppl 5):111–118. [PubMed] [Google Scholar]
- Omtzigt JGC, Los FJ, Grobbee DE, et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology. 1992;42(4, suppl 5):119–125. [PubMed] [Google Scholar]
- Wegner C, Nau H. Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 1992;42(4, suppl 5):17–24. [PubMed] [Google Scholar]
- Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42(4, suppl 5):83–88. [PubMed] [Google Scholar]
- Morrell MJ. The new antiepileptic drugs and women: efficacy, reproductive health, pregnancy, and fetal outcome. Epilepsia. 1996;37(suppl 6):S34–S44. doi: 10.1111/j.1528-1157.1996.tb06037.x. [DOI] [PubMed] [Google Scholar]
- Gram L, Jensen PK. Carbamazepine toxicity. In: Levy RH, Mattson R, Meldrum B, et al, eds. Antiepileptic Drugs. New York, NY: Raven Press. 1989 555–565. [Google Scholar]
- Hart RG, Easton JD. Carbamazepine and hematologic monitoring. Ann Neurol. 1982;11:309–312. doi: 10.1002/ana.410110312. [DOI] [PubMed] [Google Scholar]
- Sobotka JL, Alexander B, Cook BL. A review of carbamazepine's hematologic reactions and monitoring recommendations. DICP. 1990;24:1214–1219. doi: 10.1177/106002809002401214. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Vergrow MI, Riley TL. Double quotidian fever caused by carbamazepine [letter] N Engl J Med. 1980;302:1262. doi: 10.1056/nejm198005293022215. [DOI] [PubMed] [Google Scholar]
- Tohen M, Castillo J, Cole JO, et al. Thrombocytopenia associated with carbamazepine: a case series. J Clin Psychiatry. 1991;52:496–498. [PubMed] [Google Scholar]
- Mucklow J. Carbamazepine and hyponatremia. Prescriber J. 1991;31:61–64. [Google Scholar]
- Tormey WP. Mechanisms of carbamazepine-induced antidiuresis. J Neurol Neurosurg Psychiatry. 1993;56:567–569. doi: 10.1136/jnnp.56.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton TD, Jackson CW. Prophylaxis of carbamazepine-induced hyponatremia by demeclocycline in six patients. J Clin Psychiatry. 1994;55:249–251. [PubMed] [Google Scholar]
- Isojarvi JIT, Airaksinen KEJ, Repo M, et al. Carbamazepine, serum thyroid hormones and myocardial function in epileptic patients. J Neurol Neurosurg Psychiatry. 1993;56:710–712. doi: 10.1136/jnnp.56.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DP, Jefferson JW. Carbamazepine. In: Moore DP, Jefferson JW, eds. Handbook of Medical Psychiatry. St. Louis, Mo: Mosby-Year Book. 1996 493–494. [Google Scholar]
- Bertilsson L, Thompson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine 10,11-epoxide: an update. Clin Pharmacokinet. 1992;22:11–21. doi: 10.2165/00003088-198611030-00001. [DOI] [PubMed] [Google Scholar]
- Dansky LV, Rosenblatt DS, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42:32–42. [PubMed] [Google Scholar]
- Jones KL, Lacro RV, Johnson KA, et al. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661–1666. doi: 10.1056/NEJM198906223202505. [DOI] [PubMed] [Google Scholar]
- Rosa FW. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674–677. doi: 10.1056/NEJM199103073241006. [DOI] [PubMed] [Google Scholar]
- Mills JL, Simpson JL. Prospects for prevention of neural tube defects by vitamin supplementation. Curr Opin Neurol Neurosurg. 1993;6:554–558. [PubMed] [Google Scholar]
- Kuhnz W, Jager-Roman E, Rating D, et al. Carbamazepine and carbamazepine-10,11-epoxide during pregnancy and post-natal period in epileptic mothers and their nursed infants: pharmacokinetics and clinical effects. Pediatr Pharmacol. 1983;3:199–208. [PubMed] [Google Scholar]
- Nau H, Kuhnz W, Egger HJ, et al. Anticonvulsants during pregnancy and lactation: transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet. 1982;7:508–543. doi: 10.2165/00003088-198207060-00003. [DOI] [PubMed] [Google Scholar]
- USP DI Drug Information for the Health Care Professional, vol I. 19th ed. Rockville, Md: Micromedex. 1999 [Google Scholar]
- Albani F, Riva R, Baruzzi A. Carbamazepine clinical pharmacology: a review. Pharmacopsychiatry. 1995;28:235–244. doi: 10.1055/s-2007-979609. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Post RM, Worthington K. Principles of clinically important drug interactions with carbamazepine, I. J Clin Psychopharmacol. 1991;11:198–203. [PubMed] [Google Scholar]
- Ketter TA, Post RM, Worthington K. Principles of clinically important drug interactions with carbamazepine, II. J Clin Psychopharmacol. 1991;11:306–312. [PubMed] [Google Scholar]
- Brewerton TD, Kramlinger KG, and Post RM. Combination therapy with lithium and carbamazepine. In: Birch NJ, ed. Lithium: Inorganic Pharmacology and Psychiatric Use. Proceedings of the 2nd British Lithium Congress held at Wolverhampton Polytechnic, Sept. 6–9, 1987. Washington, DC: IRL Press. 1987 57–60. [Google Scholar]
- Denicoff KD, Ali SO, Smith-Jackson EE, and et al. Cognitive side effects of lithium, carbamazepine and their combination in patients with bipolar disorder. In: New Research Program and Abstracts of the 150th Annual Meeting of the American Psychiatric Association. 21May1997 San Diego, Calif. Abstract NR424:183. [Google Scholar]
- Messenheimer J, Ramsay R, Willmore L, et al. Lamotrigine therapy for partial seizure: a multicenter, placebo-controlled, double blind, crossover trial. Epilepsia. 1994;35:113–121. doi: 10.1111/j.1528-1157.1994.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Pellock JM, Rao C, Earl N. Lamotrigine efficacy and safety update: US experience [abstract] Epilepsia. 1993;34(suppl 6):42. [Google Scholar]
- Schachter SC. A multicenter, placebo-controlled evaluation of the safety of lamotrigine (Lamictal) as add-on therapy in outpatients with partial seizures. Presented at the 1992 annual meeting of the American Epilepsy Society. 4–10December1992 Seattle, Wash. [Google Scholar]
- Tomson T, Ohman I, Vitols S. Lamotrigine in pregnancy and lactation: a case report. Epilepsia. 1997;38:1039–1041. doi: 10.1111/j.1528-1157.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44(suppl 5):17–22. [PubMed] [Google Scholar]
- Richens A. Clinical pharmacokinetics of gabapentin. In: Chadwick D, ed. New Trends in Epilepsy Management: The Role of Gabapentin. London, England: Royal Society of Medicine Services. 1993 41–46. [Google Scholar]
- Boyd RA, Bockbrader HN, Turck D, and et al. Effect of subject age on the single dose pharmacokinetics of orally administered gabapentin (CI-945) [abstract]. Pharm Res. 1990 7(suppl). 215. [Google Scholar]
- Comstock TJ, Sica DA, Bockbrader HN, et al. Gabapentin pharmacokinetics in subjects with various degrees of renal function [abstract] J Clin Pharmacol. 1990;30:862. [Google Scholar]
- Halstenson CE, Keane WF, Tuerck D, et al. Disposition of gabapentin (GAB) in hemodialysis (HD) patients [abstract] J Clin Pharmacol. 1992;32:751. doi: 10.1002/j.1552-4604.1995.tb05020.x. [DOI] [PubMed] [Google Scholar]
- Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog, and man. Arzneimittelforschung. 1986;36:830–839. [PubMed] [Google Scholar]
- Busch E. Effect of Maalox TC on single dose pharmacokinetics of gabapentin capsules in healthy subjects. Pharm Res. 1992 (suppl). 315. [Google Scholar]
- Crawford P, Ghadiali E, Lane R, et al. Gabapentin as an antiepileptic drug in man. J Neurol Neurosurg Psychiatry. 1987;50:682–686. doi: 10.1136/jnnp.50.6.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndel F. Interaction of gabapentin with other antiepileptics [letter] Lancet. 1994;1:1363–1364. [PubMed] [Google Scholar]
- Calabrese JR, Shelton MD III, Keck PE Jr, and et al. Topiramate in severe treatment-refractory mania. In: New Research Program and Abstracts of the 151st Annual Meeting of the American Psychiatric Association. 2June1998 Toronto, Ontario, Canada. Abstract NR202:121. [Google Scholar]