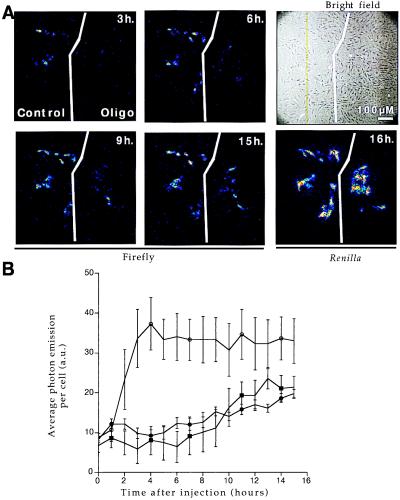
Figure 3.
RAMA 27 nuclei were comicroinjected with PGK(+)PPT/luc construct and a Renilla-expressing vector (SV40/RL) in the presence or absence of various oligonucleotides (15TCG at 1,600 μM, Acr-15TCG at 540 μM concentrations in the microcapillary). Cells were cultivated in the presence of 1 mM luciferin on the microscope stage so that firefly activity could be directly measured by photon output at regular intervals as indicated. Renilla expression was measured at the end of the experiment to determine the number of successfully microinjected cells. (A) Single-cell imaging. In the absence (control field) or presence (oligo field) of 540 μM Acr-15TCG oligonucleotide, firefly expression is shown at different times after injection: 3, 6, 9, and 15 h. Renilla expression was measured at 16 h. Bright-field image shows all of the cells in the microinjected area. (B) Quantification of firefly luciferase activity. Time course of firefly luciferase activity for different treatments: ○, control; ●, 15TCG at 1,600 μM; and ■, Acr-15TCG at 540 μM. Average photon emission per cell with integration periods of 30 min was determined by using Renilla activity to count the number of positive cells (n = 32, 19, and 18 for ○, ●, and ■, respectively). Control injections in the absence of oligonucleotide or presence of a control oligonucleotide (15-I1 at 1,600 μM) produced undistinguishable levels of firefly luciferase gene expression. Five arbitrary units (a.u.) corresponds to background photon emission level.