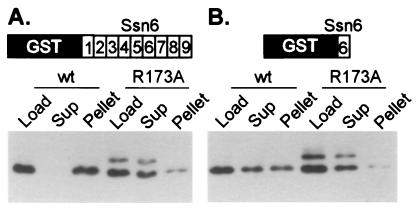
Figure 2.
An intact PTS1 sequence in the homeodomain of α2 is required for TPR binding. Wild-type or mutant (R173A) α2 extracts (Load) were incubated with resin containing immobilized GST-TPRs. After the incubation, the supernatant (Sup) contains unbound protein, and bound protein is pelleted with the resin and later eluted (Pellet). Equivalent volumes of sample were loaded in all lanes of a SDS/PAGE gel. Shown are Western blots of these gels probed with anti-α2 antibody. (A) This blot shows the result of incubating bacterial extracts containing either partially purified wild-type α2 or α2 R173A with resin containing immobilized GST:Ssn6 TPRs 1–9. (B) The resin contained immobilized GST:Ssn6 TPR6. The experiment of A was conducted at a final salt concentration of 175 mM, whereas the experiment in B was conducted at 150 mM final salt. The band of larger molecular weight present in the lanes containing α2 R173A is a covalent dimer of α2. The fraction of covalent dimer present in wild-type preparations is known to vary (39) and because the R173A protein behaves like the wild-type with respect to DNA binding and protein–protein interactions with a1 and Mcm1 (see below) we do not believe the presence of this dimer affects the interpretation of these experiments.