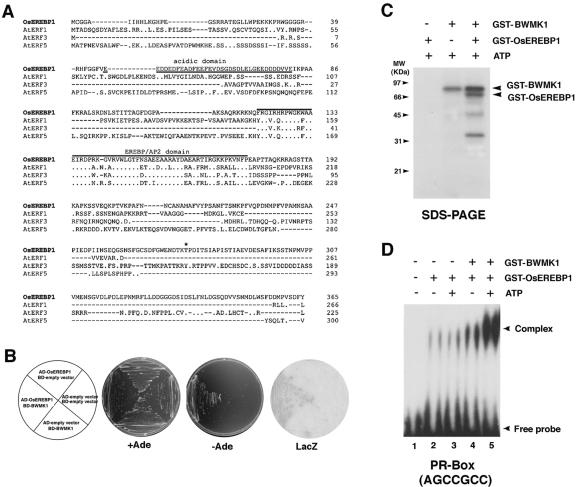
Figure 7.
BWMK1 phosphorylates OsEREBP1 and the phosphorylation enhances the ability of the transcription factor to bind to the cis-acting element. A, Alignment of the OsEREBP1 amino acid sequence with the EREBPs, AtERF1-5 (Fujimoto et al., 2000). Overlined sequences, Conserved EREBP/AP2 domain. Underlined sequences, N-terminal acidic domain. Identical residues are indicated with dots. A putative MAPK phosphorylation site is indicated by an asterisk. B, Yeast two-hybrid interaction of BWMK1 with OsEREBP1. Yeast strain pJ69-4A transformed with the constructs indicated in the left plant were grown on synthetic complete (SC) medium minus Trp and Leu (+Ade) or in SC medium minus Trp, Leu, and Ade (–Ade). β-Galactosidase activity in the colonies grown in +Ade medium (LacZ) was determined by filter-lift assay. BD, Fusion to a plasmid containing the GAL4 DNA-binding domain; AD, Fusion to a GAL4 transcriptional activation domain. C, BWMK1 phosphorylates OsEREBP1 in vitro. Arrows indicate the positions of autophosphorylated GST-BWMK1 and phosphorylated GST-OsEREBP1. The apparent molecular masses (kilodaltons) are indicated at the left. D, The DNA-binding activity of OsEREBP1 to the GCC box motif (AGCCGCC) is enhanced by BWMK1-mediated phosphorylation. The arrowheads mark the position of the protein-DNA complex and free probe.