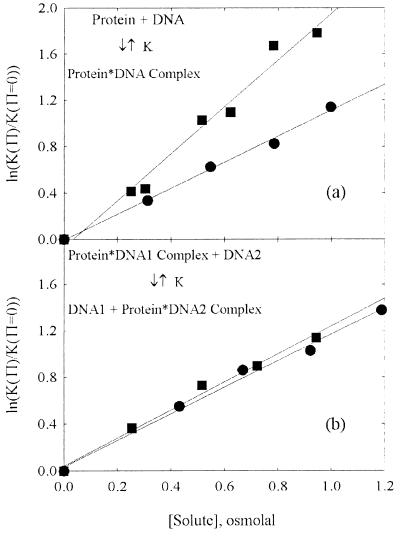
Figure 3.
The observed dependence of an osmotic stress effect on the solute nature gives insight into the basis of the exclusion. (a) The change in the binding energy of E. coli gal repressor to a DNA containing its operator sequence with increasing solute concentration is shown as a function of the osmotic pressure of the solute, in osmolal units (data taken from ref. 17). Both betaine glycine (●) and triethylene glycol (■) are apparently acting on the reaction through a difference in New between the free protein and DNA in solution and in the complex. The difference in waters, however, depends on the solute; ΔNew = 180 waters per complex for triethylene glycol and 100 for betaine glycine. This difference is characteristic of reactions that bury exposed surface area. (b) The osmotic stress dependence of the energy difference between the specific binding of the restriction nuclease EcoRI to a DNA fragment (DNA2) that contains its recognition sequence vs. the nonspecific binding of the enzyme to a DNA oligonucleotide (DNA1) that does not carry the recognition sequence. This is shown for the same two solutes, betaine glycine (●) and triethylene glycol (■) (data taken from ref. 8). In this case the effect of the two solutes is identical, within experimental error, with ΔNew = 110 waters. This result is characteristic of reactions with changes in the numbers of waters that are sequestered in sterically inaccessible pockets or cavities.