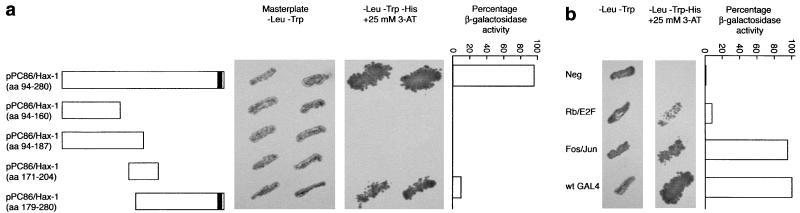
Figure 2.
The COOH terminus of Hax-1 mediates interaction with PKD2. The yeast two-hybrid system was used to determine the region in Hax-1 necessary for the interaction with loop 5 of PKD2. An interaction was assayed by prototrophy for histidine in the presence of 25 mM 3-aminotriazole and by a liquid β-galactosidase assay (the β-galactosidase activity achieved with the wild-type GAL4 protein was set at 100%). Although the last 102 aa of Hax-1 are sufficient for the association with PKD2, this interaction is not as strong as the one seen with the longer Hax-1 fragment. Therefore additional amino acids are probably necessary as part of the domain interacting with PKD2 or to maintain the appropriate conformation of Hax-1 necessary to interact with PKD2. The putative transmembrane domain of Hax-1 is depicted as a vertical bar at the COOH terminus.