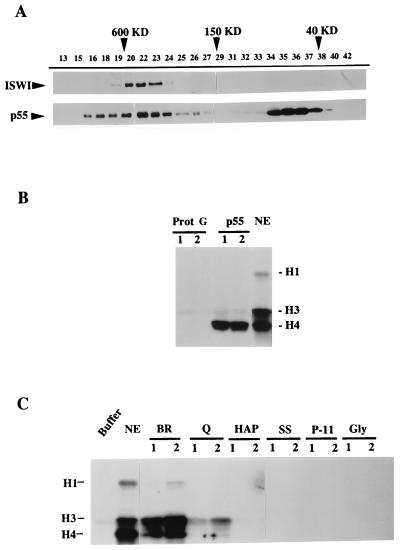
Figure 3.
p55 is associated with other proteins in Drosophila extracts. (A) Superose 6 gel filtration chromatography of Drosophila embryo S-150 extract. Fractions as indicated by numbers were analyzed by Western blotting for ISWI (Upper) and p55 (Lower). (B) Recombinant p55 interacts with a HAT activity. 20 μl of anti-Myc protein G-Sepharose beads, unbound or bound to saturating amounts of Myc epitope-tagged p55, were incubated for 3 h with Drosophila nuclear extract (NE) (19), pelleted and washed, and resuspended in 30 μl of HAT assay buffer. Bead suspension (lane 1, 5 μl; lane 2, 15 μl) and the NE (1 μl) were analyzed for HAT activity, as monitored by the transfer of the [3H]acetyl group to exogenously added histones and detection by SDS-PAGE and fluorography. Histones H1, H3, and H4 are indicated. Myc beads alone did not associate with HAT activity. (C) NURF has no HAT activity. Active NURF fractions through purification steps as in the Fig. 2A legend were assayed for HAT activity, as monitored by transfer of the [3H]acetyl group to histones and detection by SDS-PAGE and fluorography.