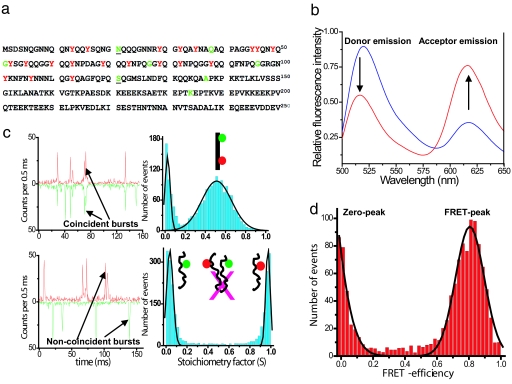
Fig. 1.
Amino acid sequence, ensemble, and single-molecule data for NM. (a) Sequence of the prion domain (NM) of Sup35 showing residues mutated to cysteine in green for single fluorescence labeling (Alexa Fluor 488). Underscores (21 and 121) indicate the dual cysteine mutation for donor (Alexa Fluor 488) and acceptor (Alexa Fluor 594) labeling for FRET studies. Tyrosines are shown in red. (b) Steady-state ensemble fluorescence spectra of double-labeled NM (0.1 μM) showing energy transfer under denatured (6 M GdmCl) conditions (blue) and under native condition (red) obtained by exciting the donor (λex 488 nm). (c) Two-color single-molecule fluorescence coincidence: Coincident bursts and stoichiometric factor histogram for dual-labeled DNA (non-FRET) standard sample (Upper) and for a mixture of two single-labeled NM (100 pM each) under native condition showing no intermolecular association under this condition (Lower). (d) Single-molecule FRET-efficiency histogram measured ratiometrically for NM under native conditions. Black curves are the best fits using Gaussian functions. See SI Text for details.