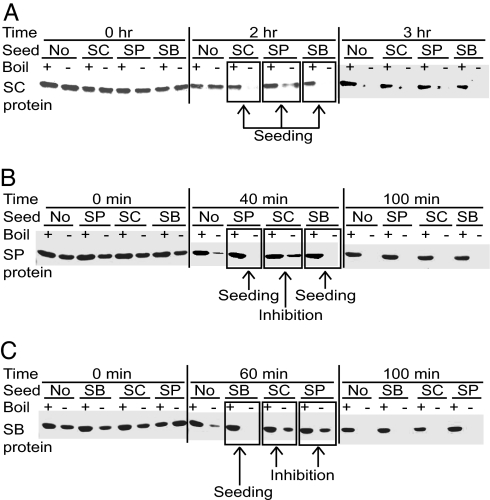
Fig. 4.
In vitro polymerization of the S. sensu stricto Sup35NM protein fragments. The purified (His)6-tagged Sup3NM regions of S. cerevisiae (Sup35NMSC or SC, A), S. paradoxus (Sup35NMSP or SP, B), and S. bayanus (Sup35NMSB or SB, C) spontaneously polymerize in the nondenaturing conditions after a certain lag period, as detected by a decrease of the monomeric fraction remaining soluble in SDS and capable of entering the SDS/PAGE gel without boiling. Boiled samples where all protein enters the gel are shown in each case as controls. Addition of preformed polymers at the ratio of 1:20 leads to the following results: Sup35NMSC promotes polymerization of Sup35NMSC (A) and Sup35NMSP (B) but delays polymerization of Sup35NMSB (C); Sup35NMSP promotes polymerization of Sup35NMSP (B) but delays polymerization of both Sup35NMSC (A) and Sup35NMSB (C); Sup35NMSB promotes polymerization of both Sup35NMSC (A) and Sup35NMSB (C) but delays polymerization of Sup35NMSP (B).