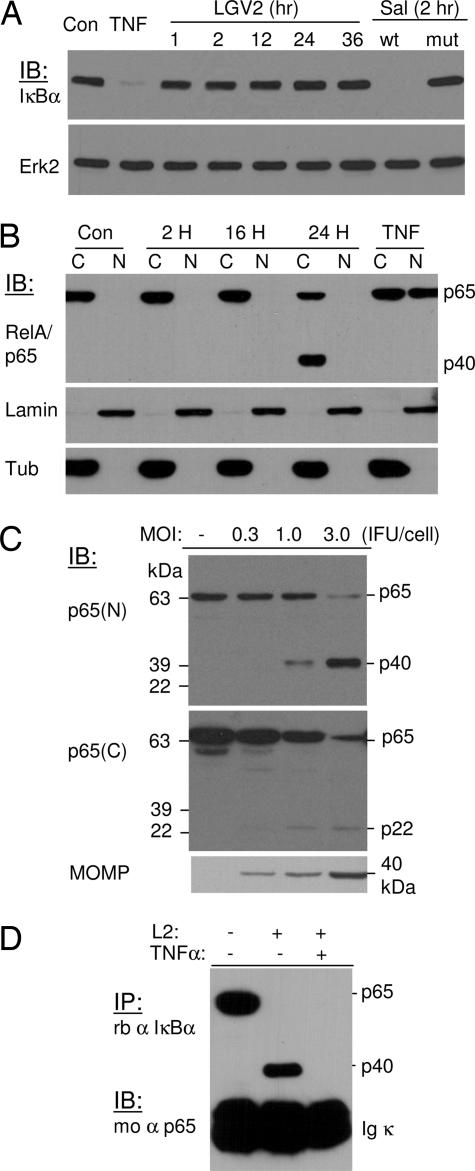
Fig. 1.
C. trachomatis infection does not induce IκBα degradation; instead, it promotes cleavage of p65/RelA. (A) HeLa 229 cells were infected with C. trachomatis LGV2 at 1 IFU per cell for various times or with invasive (wt) or invasion-defective (mut) S. typhimurium for 2 h. TNF-α at 10 ng/ml was used as a positive control for IκBα degradation. The expression and degradation of IκBα were detected by immunoblotting analyses. Erk2 expression was used as a loading control. (B) HeLa 229 cells were infected with LGV2 at 1 IFU per cell for 2, 16, and 24 h. The cytosolic (C) and nuclear (N) fractions were separated, and p65/RelA redistribution was detected by blotting analysis. The expression of lamin A and β-tubulin were used as loading controls for nuclear and cytosolic fractions, respectively. Chlamydia infection does not promote nuclear translocation. In addition, a cleavage product of 40 kDa (p40) was detected from the cytosolic fraction of a sample of late infection. (C) Whole-cell lysates from infected and uninfected control cells were separated by SDS/PAGE, and cleavage of p65 was detected with an N or C terminus-specific antibody against p65. A 40-kDa (p40) protein band and a 22-kDa (p22) protein band were detected from samples infected for 28 h with the N and C terminus-specific antibody, respectively. The production of MOMP was monitored as an indication of infection. (D) Association of p40 with IκBα protein. HeLa 229 cells were infected with Chlamydia LGV2 at a multiplicity of infection of 3 for 28 h. The cells were then treated with 10 ng/ml TNF-α for 15 min to induce IκBα degradation, or the cells remained untreated. Lysates from infected and uninfected samples were immunoprecipitated with an anti-IκBα antibody. The association of wild-type p65 or the cleavage product with IκBα was detected by immunoblotting. Both wild-type p65 and p40 associate with IκBα. Depletion of IκBα with TNF-α treatment abolishes the association of p40 with IκBα.