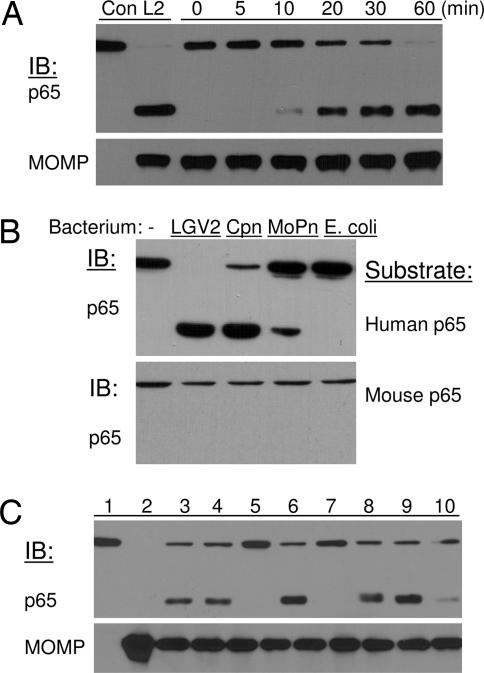
Fig. 4.
Purified Chlamydia elementary bodies contain p65 cleavage activity. (A) Gradient-purified EBs were solubilized with a buffer containing 1% Nonidet P-40. One microgram of soluble bacterial proteins was incubated with p65 from 293T cells at 37°C for various times as indicated. (Upper) The two lanes on the far left, labeled con and L2, represent samples from uninfected and LGV2 infected cells, respectively. (Lower) The content of MOMP was used as a measure of EB input. (B) Soluble proteins from different Chlamydia species contain p65 cleavage activity against human, but not mouse, p65 proteins. (C) Treatment with serine protease inhibitors blocks p65 cleavage activity. In parallel experiments, protease inhibitors were included in the in vitro p65 cleavage assay. Lanes: 1, 293T lysate; 2, solubilized EB; 3, p65 and EB proteins incubated at 37°C for 60 min; 4, Roche protease inhibitor mixture at 10×; 5, heat-inactivated EB; 6, EDTA at 5 mM; 7, lactacystin at 10 μM; 8, MG132 at 20 μM; 9, tosylphenylalanylchloromethane at 20 μM; 10, tosyl-lysylchloromethane at 20 μM. MOMP was used as a loading control.