Figure 2.
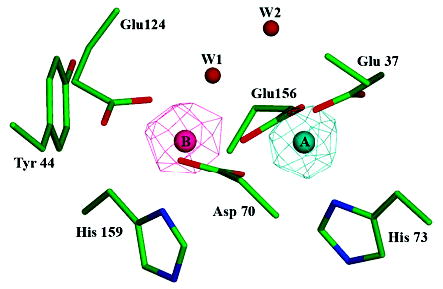
Bacterioferritin-like dimetal binding site. Residues Glu37, Asp70, His73, Glu124, Glu156, and His159 of SsDPSL form a dimetal binding site within the core of the four-helix bundle. Side chain atoms are colored in green (carbon), blue (nitrogen), and red (oxygen). Anomalous difference electron density maps (mesh) indicate asymmetric binding of the iron and zinc atoms, with Zn2+ binding preferentially to the A site (cyan) and Fe3+ to the B site (pink). The iron edge anomalous difference map is contoured at 8 σ (pink mesh), whereas the zinc edge anomalous difference map is contoured at 15 σ (cyan mesh). When contoured at lower levels, however, it is clear that each site contains a mixed population of both iron and zinc. Water W1 shows strong coordination to the B site metals but only weak coordination, at best, to the A site.
