Figure 1.
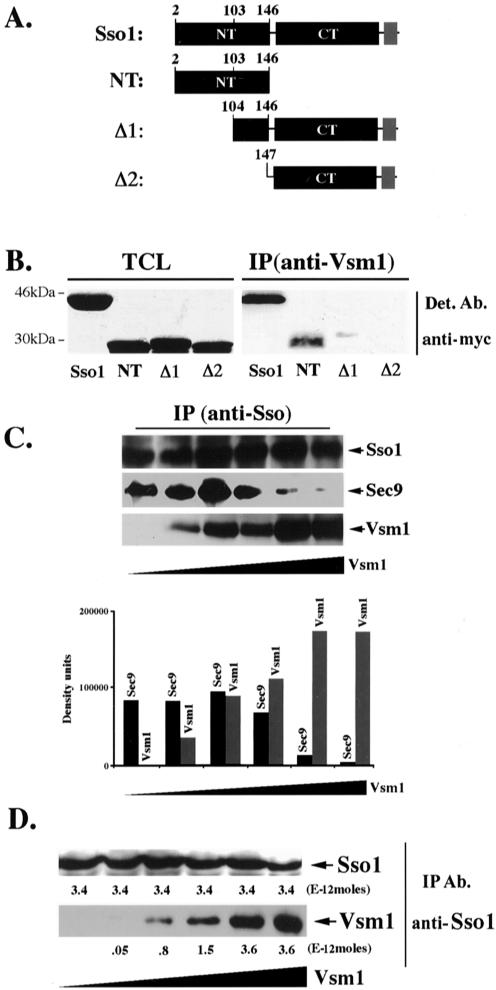
Vsm1 binds directly to the Sso1 t-SNARE. (A) Scheme of Sso1 deletion mutants. Native Sso1 and deletion mutants (e.g., Sso12-146 [NT], Sso1Δ1-103 [Δ1], and Sso1Δ1-146 [Δ2]) are depicted schematically. NT indicates the NH2-terminal autoinhibitory domain, and CT indicates the COOH-terminal SNARE binding domain and flanking region. The transmembrane domain of Sso1 is shown in gray. Proteins were tagged at the NH2 terminus by using the myc epitope. (B) The NH2 terminus of Sso1 is required for Vsm1 binding. WT cells producing myc-tagged Sso1, Sso12-146, Sso1Δ1-103 and Sso1Δ1-146 were lysed and subjected to IP with anti-Vsm1 abs. Immunoprecipitated complexes were resolved on the gels and detected (Det.) with anti-myc and anti-Vsm1 abs (latter not shown). TCL (100 μg of protein). (C) Vsm1 displaces Sec9 binding to Sso1. GST-Sso11-265 and GST-Sec9402-651 (2E-11 and 1E-11 moles, respectively) were mixed with increasing amounts of His6-Vsm1 (between 0.2 and 10E-11 moles) and incubated overnight (o.n.) at 4°C (see MATERIALS AND METHODS). Proteins were immunoprecipitated with anti-Sso abs and detected in blots. (D) The stoichiometry of Vsm1-Sso1 binding is 1:1. Moles (2.3E-11) of GST-Sso11-265 were mixed with increasing amounts of His6-Vsm1 (between 1 and 16E-11 moles) and incubated overnight at 4°C. Proteins were immunoprecipitated with anti-Sso abs and detected in blots. Molar quantification of the proteins was determined using known amounts of GST-Sso11-265 and His6-Vsm1 that were electrophoresed and detected in parallel to the immunoprecipitated proteins. The stoichiometry is defined as the ratio of the number of moles of GST-Sso11-265 (3.4E-12 moles) immunoprecipitated to the number moles of His6-Vsm1 (3.6E-12 moles) coimmunoprecipitated at saturation.