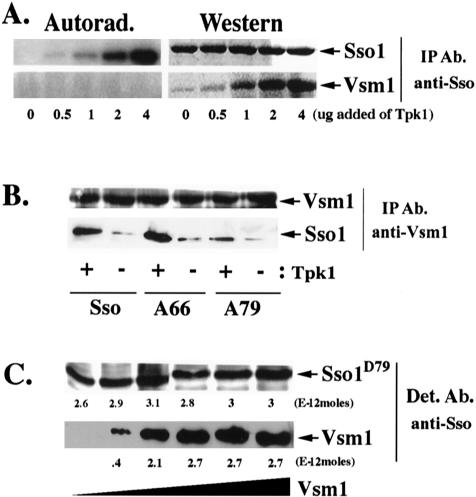
Figure 2.
Phosphorylation of Sso1 at serine-79 enhances Vsm1 binding in vitro. (A) PKA-dependent phosphorylation of Sso1 increases the Vsm1 binding in vitro. GST-Sso11-265 and His6-Vsm1 (3.4E-11 moles each) were mixed with increasing amounts of Tpk1 in the presence of either ATP or [γ-32P]ATP for 1 h at 30°C. Proteins were immunoprecipitated with anti-Sso abs and detected in blots by using anti-Sso and -Vsm1 abs or by autoradiography. (B) PKA-dependent phosphorylation of Sso1 at serine-79 is important for the Vsm1 binding in vitro. Phosphorylated and nonphosphorylated GST-tagged native Sso11-265 (Sso) and alanine substitution mutants Sso11-265,A66 (A66) and Sso11-265,D79 (A79) were incubated overnight at 4°C with His6-Vsm1 at a 1:1 molar ratio. Proteins were immunoprecipitated using anti-Vsm1 abs and were detected in blots with anti-Sso or -Vsm1 abs. (C) The stoichiometry of Vsm1 and Sso1D79 binding is 1:1. 2.3E-11 moles of GST-Sso11-265,D79 were mixed with increasing amounts of His6-Vsm1 (between 1 and 16E-11 moles) and incubated overnight at 4°C (see MATERIALS AND METHODS). Molar quantification of the proteins was determined using known amounts of GST-Sso11-265,D79 and His6-Vsm1 that were detected in parallel. The stoichiometry was defined as the ratio of the number of moles of GST-Sso11-265,D79 (3E-12 moles) immunoprecipitated to the number moles of His6-Vsm1 (2.7E-12 moles) coimmunoprecipitated at saturation.