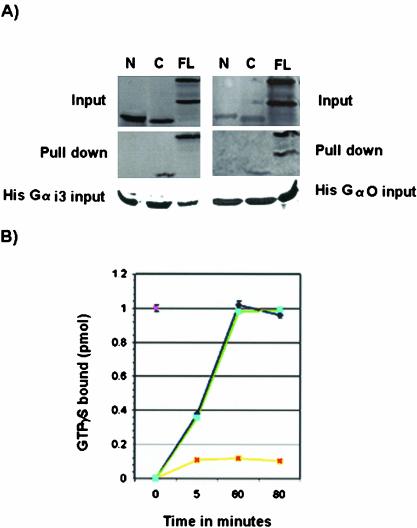
Figure 8.
GDI activity of mouse LGN. (A) Binding of mouse LGN to Gα subunits of heterotrimeric G-proteins. In vitro–translated LGN constructs containing amino acids 1–384 from the N-terminus (N), amino acids 385–670 from the C termini (C), or full length (FL) were incubated with His-Gαi3 or His-Gαo bound to His columns. Only FL and C products copurify with His-Gαi3/o. No binding is detected between construct N and Gαi3 or Gαo. In the FL lane, two bands are detected, an upper band corresponding to LGN and a lower band being a byproduct of the translation reaction. (B) GDI activity of mouse LGN on Gαi3. Time course experiments showing the rate of [35S]GTPγ binding by Gαi3 were carried out in the presence of 1 μM GST-LGN (yellow). Control experiments using GST alone are shown in blue. GST-LGN inhibits ∼90% of [35S]GTPγ binding to Gαi3 but the GST control has no effect. The effect of LGN on Gαi3 activity is observed as early as 5 min and lasts >80-min time period. GST-LGN effect on Gαo (green) is similar to the GST control (blue), indicating mouse LGN has no GDI activity on Gαo.