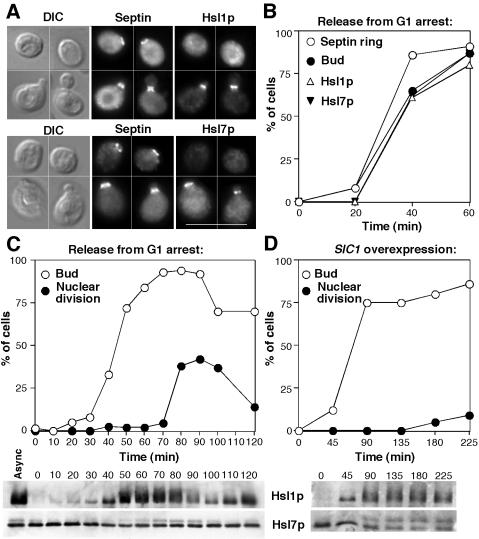
Figure 3.
Timing of Hsl1p and Hsl7p localization and phosphorylation. (A) Asynchronous cells of strain DLY5000 (HSL1-myc HSL7-HA) were double stained to visualize the septin Cdc11p and either Hsl1p-myc (top) or Hsl7p-HA (bottom). Bar, 10 μm. (B and C) Cells of strain DLY5000 were synchronized by pheromone-induced G1 arrest/release in two separate experiments. (B) Aliquots of one culture were fixed and stained to examine the septin ring formation, Hsl1p recruitment, Hsl7p recruitment, and bud formation, as indicated. More than 200 cells were scored for each sample. (C) Aliquots of the other culture were lysed, separated by SDS-PAGE, and immunoblotted to detect Hsl1p-myc (top) or Hsl7p-HA (bottom). Parallel aliquots were fixed, stained with 4,6-diamidino-2-phenylindole to visualize DNA, and scored for bud emergence and nuclear division (n = 200). (D) Cells of strain DLY5777 (HSL1-myc HSL7-HA GAL1p-SIC1) were grown to exponential phase in sucrose-containing medium and arrested in G1 with pheromone. Galactose was added to 2% final concentration to induce Sic1p expression (this strain contains multiple integrants of the GAL1p-SIC1 construct, which produce enough Sic1p to block cell cycle progression), and 1 h later the cells were washed and resuspended in galactose-containing medium lacking pheromone. All operations were performed at 30°C. Bud emergence, nuclear division, and Hsl1p and Hsl7p phosphorylation were then assessed after release from arrest.