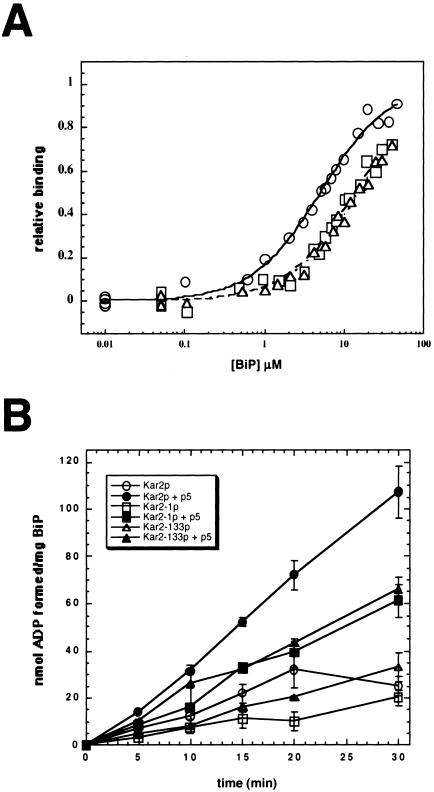
Figure 7.
Kar2-1p and Kar2-133p exhibit reduced peptide affinity and interdomain coupling. (A) Fluorescence anisotropy measurements of F-APPY binding to wild-type BiP (○), Kar2-1p (□), and Kar2-133p (▵), and a best-fit analysis of the data using a single binding site was performed as described by Montgomery et al. (1999). (B) Steady state ATPase assays were performed after assembling reactions on ice in either the presence (filled symbols) or absence (open symbols) of a 1000-fold molar excess of p5: Wild-type BiP (circles), Kar2-1p (squares), and Kar2-133p (triangles).