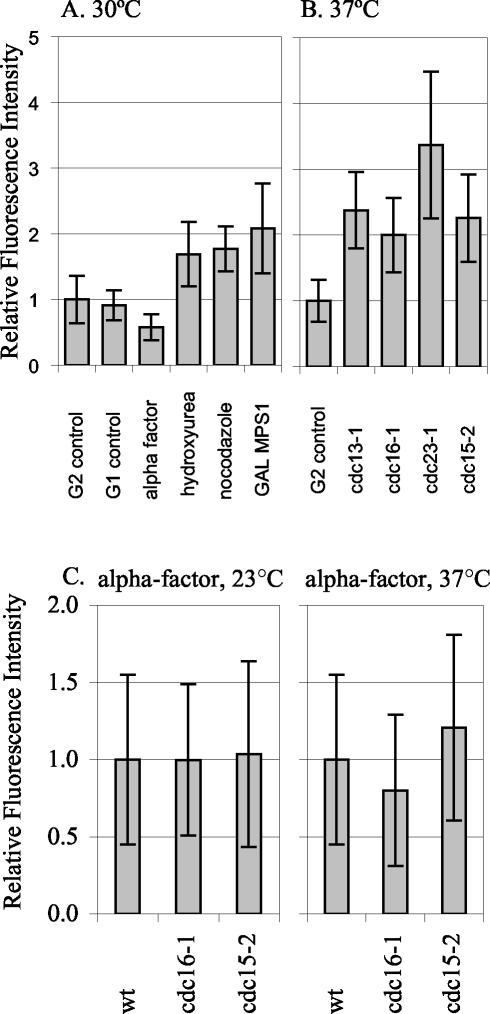
Figure 5.
Spc110p-YFP at the SPB during different cell cycle arrests. The relative amount of Spc110p-YFP at the SPB in control and arrested cells was quantified. (A) Arrests at 30°C. G2 control: SPBs from TYY80-5A cells in G2 with separated SPBs and short spindles were quantified. The mean intensity of the 30°C G2 control, was set as 1.0. G1 control: SPBs from unbudded TYY80-5A cells with a single SPB spot were quantified. α-Factor: strain TYY80-5A was arrested in α-factor (see MATERIALS AND METHODS). The measurement of Spc110p at the SPBs in cells treated with α-factor was performed eight times in three different strains. A representative experiment is shown. Hydroxyurea: strain DHY15 was grown to early logarithmic phase in YPD and arrested by adding hydroxyurea to a final concentration of 0.1 M, cells were collected after 180 min. Fluorescence intensity in hydroxyurea is shown relative to DHY15 G2 control. Nocodazole: strain TYY80-5A was grown to early logarithmic phase in YPD and arrested by adding nocodazole to a final concentration of 15 μg/ml, cells were collected after 140 min. Because nocodazole collapses the spindle, the two SPBs appeared as a single spot, and the total intensity was divided by 2 to get the average intensity per SPB. GAL MPS1: strain TYY102-5D was grown in YPR and arrested by adding 2% galactose to an early logarithmic phase culture, cells were collected after 195 min. (B) Arrests at 37°C. G2 control: strain TYY80-5A was grown at 37°C and SPBs from cells in G2 with separated SPBs and short spindles were quantified. The mean intensity of the 37°C G2 control was set as 1.0. cdc13-1, cdc16-1, cdc23-1, and cdc15-2: strains TYY92-18B, TYY93-16D, TYY110, and TYY109, respectively, were arrested at the nonpermissive temperature (37°C) for 195 min. (Although four of the strains contain MET3-SPC110-YFP, it was not induced for these experiments. Instead all strains were grown in YPD [or YPR] medium supplemented with 300 μg/ml methionine to repress MET3 SPC110-YFP.) (C) Temperature shifts in G1. Wild-type, cdc16-1, and cdc15-2 cultures were arrested in G1 with α-factor at 23°C. After 1.5 generations, the culture was filtered and resuspended in media with fresh α-factor and shifted to 37°C for 90 min. Under these conditions the arrest was maintained, as monitored by fluorescence-activated cell sorting analysis.