Figure 4.
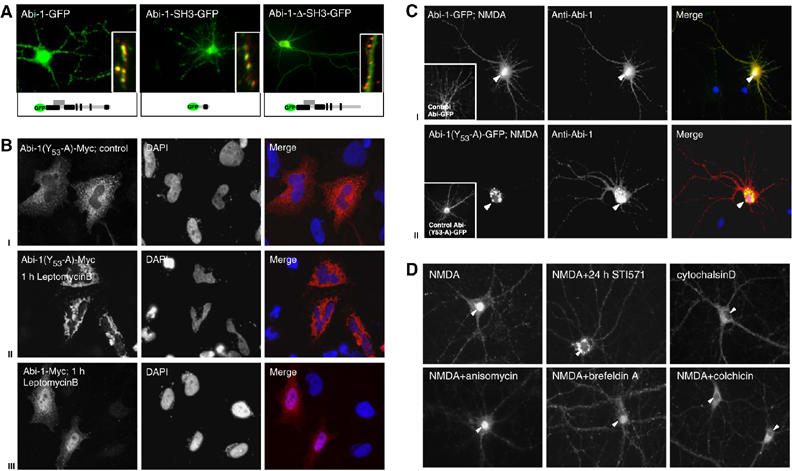
Determinants of Abi-1 targeting to the nuclear and synaptic compartments. (A) Full-length Abi-1-GFP protein and the Abi-1 SH3 domain colocalize with the presynaptic marker protein Bassoon (red, insets). Deletion of the SH3 domain results in the loss of synaptic targeting. (B) Phosphorylation of Abi-1 tyrosine 53 is a prerequisite for nuclear import leptomycinB treatment of Abi-1-(53Y-A)-Myc-transfected HeLA cells (localized in the cytoplasm under control conditions, I) leads to accumulation of the protein in the perinuclear area (II). Wild-type Abi-1-Myc (III) is enriched in the nucleus after leptomycinB treatment. (C) After NMDA application to hippocampal neurons, full-length Abi-1-GFP as well as the endogenous Abi-1 (red) readily translocates into the nucleus (yellow staining, I). Transfected Abi-1 protein, mutated at tyrosine 53 (Abi-1-(53Y-A)-GFP), accumulates after NMDA application in the perinuclear area. Endogenous Abi-1 (red) is enriched in the nucleus (II). (D) Inhibition of c-Abl by the specific compound STI571 for 24 h before NMDA treatment results in a perinuclear accumulation and no nuclear enrichment of Abi-1 (compare B and C). Nuclear translocation can also be prevented by the disturbance of cytoskeletal components with colchicin or cytochalasinD. The application of anisomycin or brefeldinA does not influence nuclear accumulation.
