Figure 6.
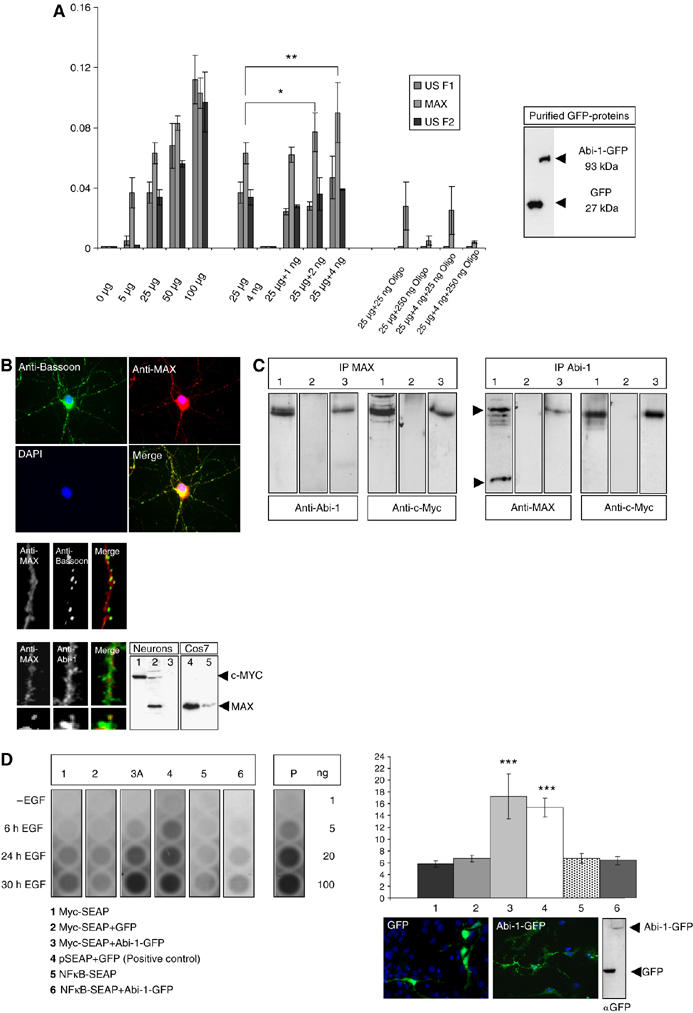
Abi-1 binds to the Myc/Max complex and enhances E-box-regulated gene transcription. (A) Concentrations of bound transcription factors from HeLa cell nuclear extracts were determined after binding to oligonucleotides with the respective recognition sequence (Transfactor kit, ELISA-based). A total of 24 different transcription factors were tested. Here, only the results for the E-box binding proteins USF1, USF2 and Max are depicted. The first cluster of bars indicates the increasing amounts of transcription factors relative to the total amount of nuclear extract measured (5–100 μg). The next cluster shows the values for 25 μg HeLa nuclear extract and 4 ng of purified Abi-1-GFP protein (also seen on Western blot). Then the values for the transcription factors after coincubation of 25 μg nuclear extract with increasing amounts of Abi-1-GFP protein are given. The relative amount of bound Max protein significantly increased by the coincubation of Abi-1-GFP. The last cluster of bars shows the internal control values after coincubation with unbound competing oligonucleotides. (B) Myc (lane 1) and Max (lanes 2 and 4, Cos7 cells as a positive control) are expressed in neurons and are mainly localized within the nucleus. Some Max-positive signals were also detected in the cytoplasm and within the dendritic compartment partly overlapping with Abi-1 and Bassoon stainings. Preabsorption of the Max antibody with the immunizing peptide results in the loss of signal (lanes 3 and 5). (C) Co-IP from nuclear extracts after stimulation (lane 1, input; lane 2, IgG control; lane 3, IP, 5 μg of protein loaded). Complexes that are precipitated with a Max antibody contain the Abi-1 protein. As a control, we stained also against Myc showing the Myc/Max highly stable complex at a size of about 63 kDa. On the other hand, experiments using Abi-1 antibodies result in the detection of the Myc/Max complex as consecutively shown by Myc staining. (D) E-box promoter assay. Neuronal cells were transfected/cotransfected with Myc-SEAP (secreted alkaline phosphatase vector carrying the E-box element), GFP-Abi-1 (lane 3) and pEGFP (lane 2). In addition, we tested NFκB-SEAP (coding for a NFκB-responsive promotor) (lane 5), NFκB-SEAP/GFP-Abi-1 (lane 6) as well as Myc-SEAP alone (lane 1) as negative control and the vector pSEAP with pEGFP (lane 4) as positive control (as recommended and provided by the manufacturer). Chemiluminescence reaction was controlled by the measurement of different amounts of purified alkaline phosphatase (AP, given in ng). Transfection rates were tested by counting cotransfected cells and Western blotting as shown. Cells were stimulated by EGF (20 ng/μl) as recommended by the supplier of the vector kit. Compared with controls (lanes 1, 2, 5 and 6), secreted SEAP was significantly elevated to levels of the positive control vector (lane 4) after 24 and 30 h EGF treatment when Abi-1-GFP was cotransfected with the Myc SEAP vector (lane 3).
