Figure 1.
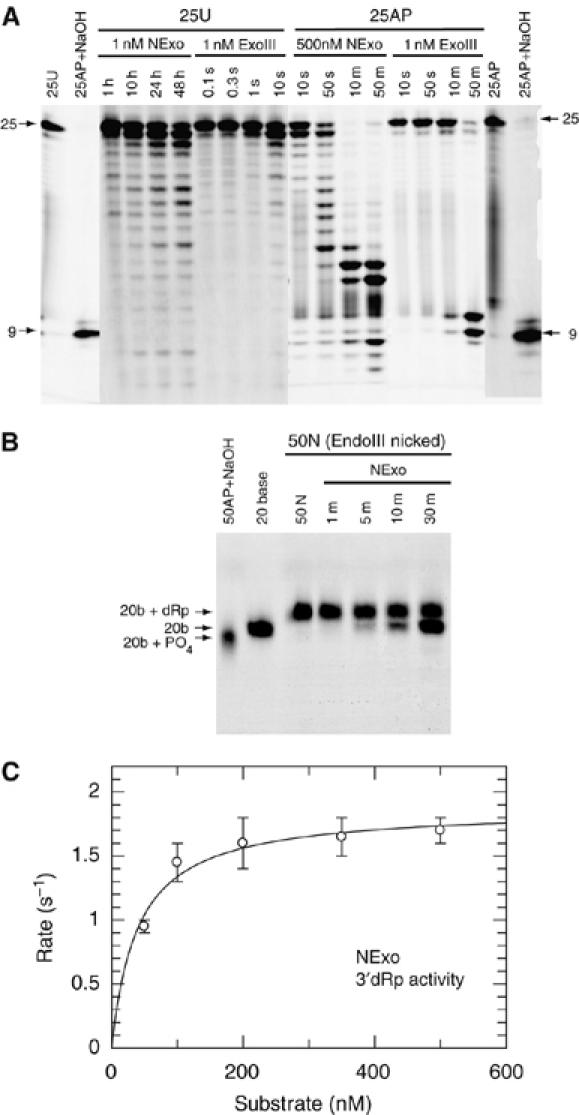
Biochemical activity of the N. meningitidis protein NExo (NMB0399). (A) Substrates 25U (100 nM) and 25AP (100 nM) were incubated with the indicated concentrations of NExo (NMB0399) or ExoIII and the products separated by denaturing PAGE. Control lanes show the unreacted 25U and 25AP substrates and the 25AP substrate after treatment with NaOH. (B) A nicked substrate with a 3′-dRp (50N) was generated by reacting a 50 bp DNA duplex with a U at position 21 with UDG (to give 50AP) and then Endonuclease III (50N). The 50N substrate (100 nM) was then reacted with 0.1 nM NExo (NMB0399) for the time periods shown before separation by denaturing PAGE. NExo is able to efficiently remove the 3′-dRp moiety from the 50N substrate before the further removal of nucleotides by exonuclease activity. Control lanes show the 50N substrate, 50AP after treatment with NaOH and a 20-base oligonucleotide. (C) The rate of 3′-dRp activity was measured for NExo by the appearance of products from the end processing of the 50N substrate. Reactions were performed as described in Materials and methods with substrate concentrations as shown and NExo at 1000-fold less. Data are shown with the best fit to the Michaelis–Menten equation: NExo has a kcat of 1.9±0.09 s−1 and KM of 40±9 nM.
