Abstract
This study was concerned with the single-dose, pharmacokinetics of cefmenoxime after intramuscular (i.m.) injections of 250, 500 and 1,000 mg; 1-h intravenous (i.v.) infusions of 500, 1,000, and 2,000 mg; and 5-min i.v. injections of 500, 1,000, and 2,000 mg of cefmenoxime. A total of 15 subjects were used, each receiving all three doses for one route of administration. Mean calculated peak plasma levels after the 250-, 500-, and 1,000-mg i.m. doses were 9.07, 14.68, and 26.73 micrograms/ml, respectively, occurring about 40 min after dosing. The biphasic decline in plasma levels after i.v administration was usually not apparent after i.m. dosing, because absorption of the drug from the injection depot was slower than distribution of the drug. Mean calculated peak levels from the 500-, 1,000-, and 2,000-mg i.v. doses were 22.8, 41.6, and 94.5 micrograms/ml, respectively, after the 1-h infusions and 64.1, 100.9, and 198.2 micrograms/ml, respectively after the 5-min injections. Small but statistically significant trends of decreasing alpha and increasing volume of distribution (central compartment) with increasing dose size were noted; however, this distribution phenomenon was self-compensating, resulting in no overall effect on plasma clearance. For practical purposes, the pharmacokinetics were linear. The mean 0- to 24-h urinary recoveries of cefmenoxime after the i.m. injections, i.v. infusions, and i.v. injections were 72.1, 67.5, and 74.5% respectively. Overall, the pharmacokinetics of cefmenoxime were best described by a two-compartment open model with a beta-phase half life of 0.91 h. Plasma clearance of the drug was dosage level and route independent, averaging 254 ml/min; thus, there was an excellent linear relationship between the area under the plasma level curve and the dose. The results of this study indicated that most of the drug is removed by renal mechanisms, with tubular secretion predominating.
Full text
PDF
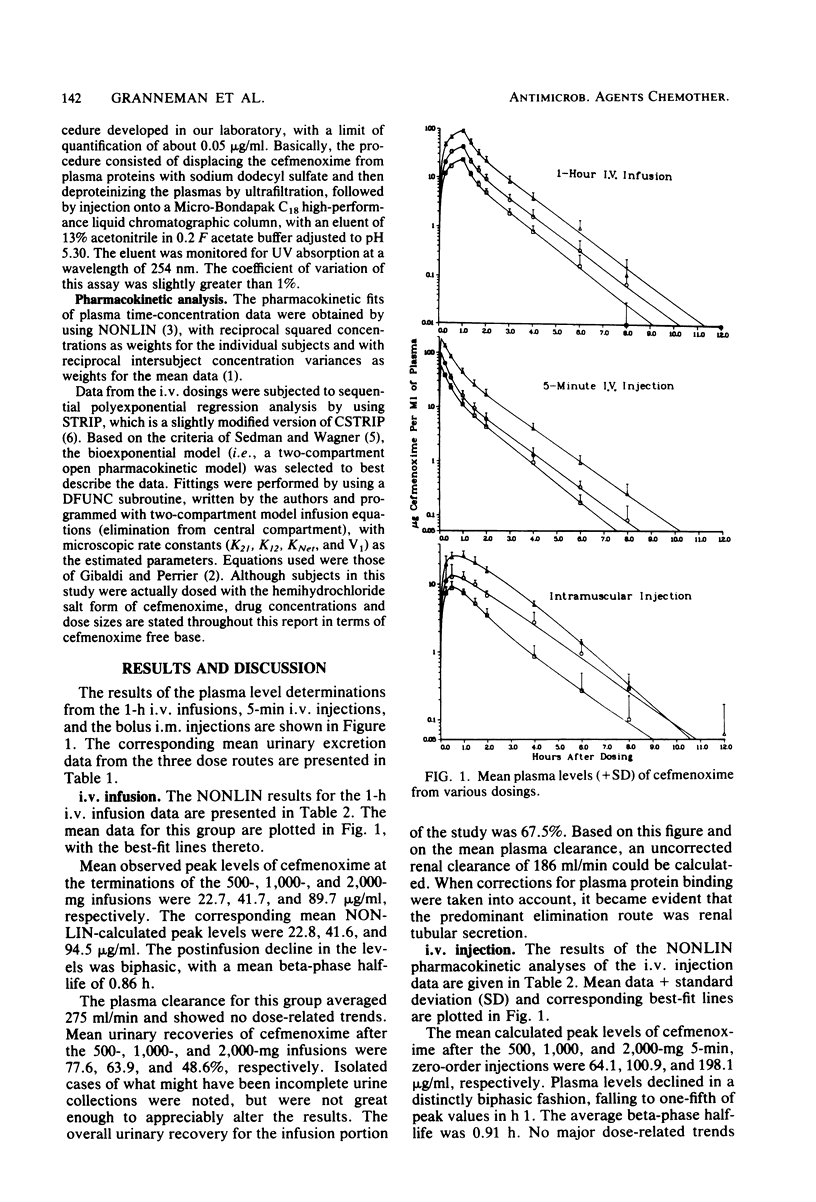
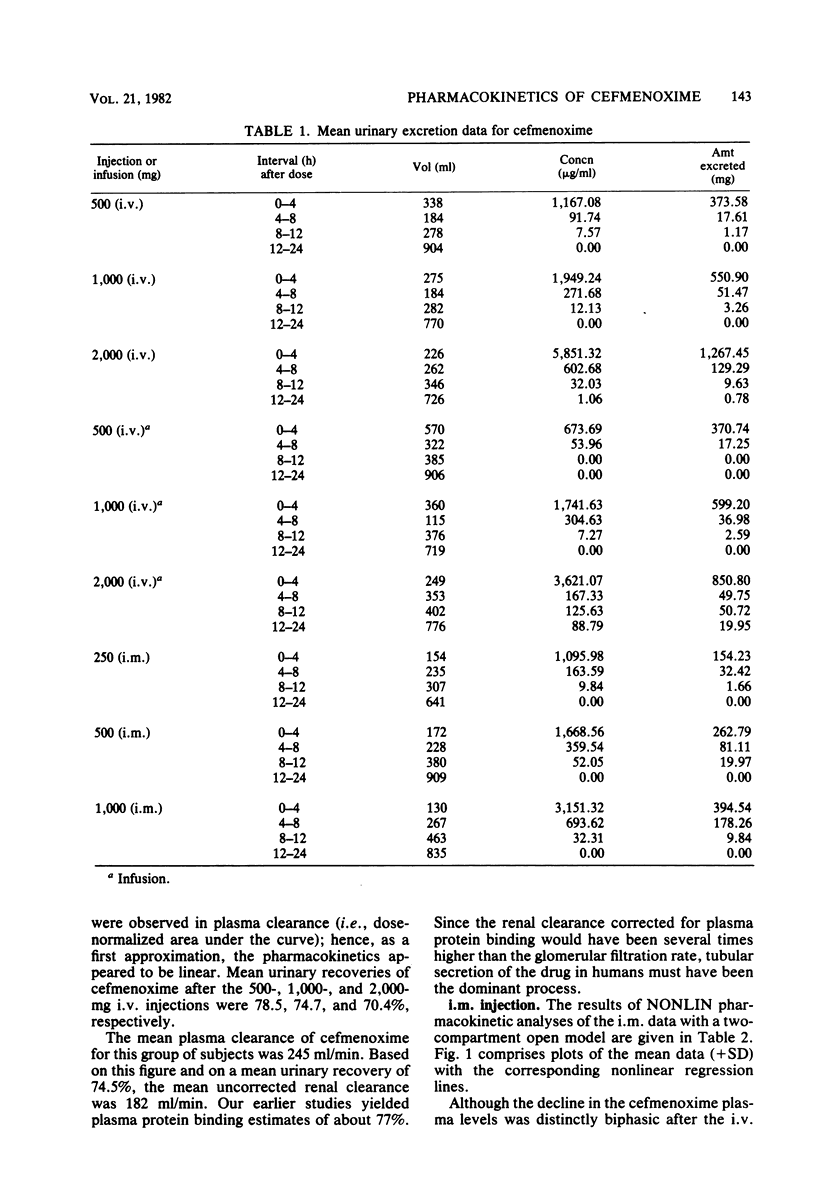
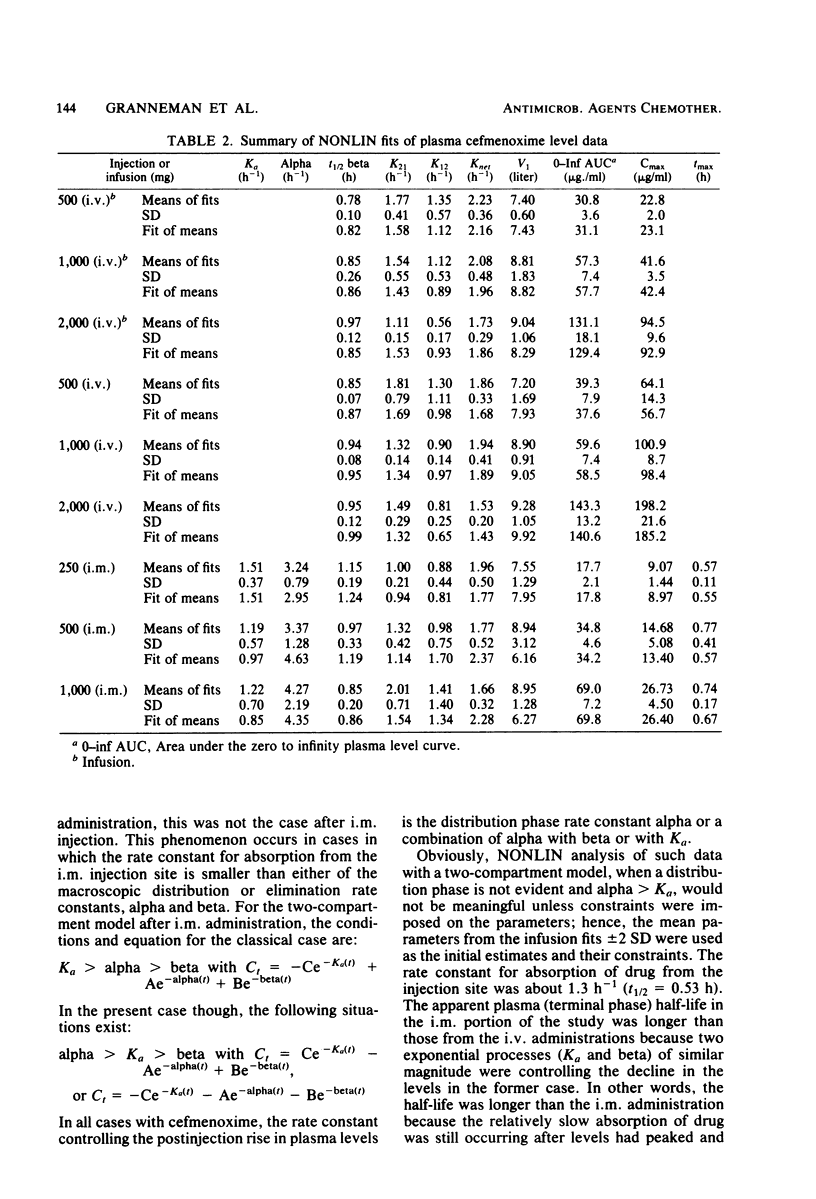

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Ochiai M., Aki O., Morimoto A., Okada T., Matsushita Y. New cephalosporin derivatives with high antibacterial activities. Chem Pharm Bull (Tokyo) 1977 Nov;25(11):3115–3117. doi: 10.1248/cpb.25.3115. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Stamm J. M., Girolami R. L., Shipkowitz N. L., Bower R. R. Antimicrobial activity of cefmenoxime (SCE-1365). Antimicrob Agents Chemother. 1981 Mar;19(3):454–460. doi: 10.1128/aac.19.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]


