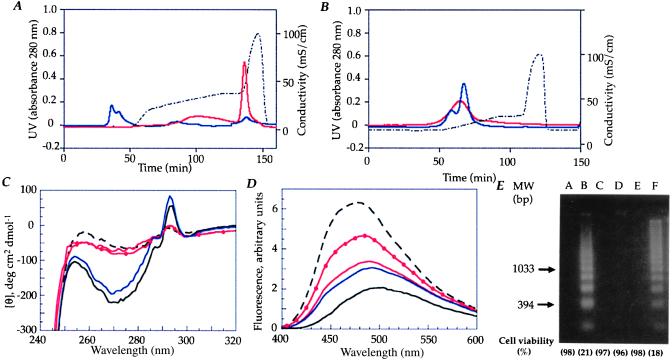
Figure 2.
Conversion of α-lactalbumin to the apoptosis-inducing form requires partial unfolding of the protein and the presence of a cofactor. (A) Apo α-lactalbumin was subjected to ion-exchange chromatography on a column previously exposed to human milk casein. More than 95% of the protein was retained on the column and eluted with 1 M NaCl (solid, red line). Native α-lactalbumin was not retained (solid, blue line). The NaCl gradient is shown by the dotted line. (B) Apo α-lactalbumin (solid, red line) and native α-lactalbumin (solid, blue line) eluted in the void volume when subjected to ion-exchange chromatography on a clean column. (C) CD spectra of the apo α-lactalbumin eluate (solid, red line) from the casein-conditioned matrix did not differ from the apo α-lactalbumin control (dashed, black line). The active fraction from casein was used as a control (solid, red line with solid circle). Native α-lactalbumin had similar spectra before (solid, black line) and after (solid, blue line) elution from the column. (D) ANS spectra of the apo α-lactalbumin eluate (solid, red line) from the casein-conditioned matrix did not differ from the apo α-lactalbumin control (dashed, black line). The native control did not bind ANS before (solid, black line) or after elution from the casein-conditioned matrix (solid, blue line). The active fraction from casein was used as a control (solid, red line with solid circle). (E) Loss of viability and DNA fragmentation of L1210 cells. Lanes: A, cell culture medium; B, the active fraction from human milk casein (0.2 mg/ml); C, native α-lactalbumin (1.0 mg/ml); D, void peak vol from the clean matrix (1.0 mg/ml); E, native α-lactalbumin eluate from the casein-conditioned matrix (1.0 mg/ml); and F, apo α-lactalbumin eluate from the casein-conditioned matrix (0.2 mg/ml). The proteins in lanes B and F were active.