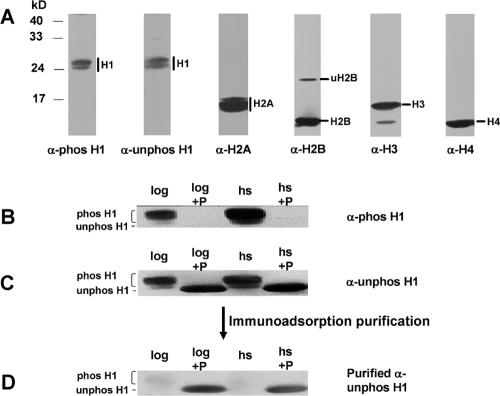
FIG. 2.
Specificity and purification of anti-H1 antibodies. A. Macronuclei were isolated as described previously (26) from heat-shocked (40°C for 1 h) cells. A total of 3 × 106 macronuclei/lane were subjected to SDS-12% PAGE, transferred to PVDF membranes, and probed with antisera that were produced against phosphorylated H1 (phos-H1), unphosphorylated H1 (unphos-H1), or H2A. Blots were stripped and reprobed with anti-H2B, -H3, or -H4 antisera. B, C, and D. Histone H1 was extracted with 5% PCA from WT cells in log phase (log) or cells heat shocked at 40°C for 10 min (hs). Aliquots of H1 were treated with λ-protein phosphatase (+P). H1 samples were subjected to SDS-PAGE, transferred to a PVDF membrane, and then probed with antisera produced against phosphorylated or unphosphorylated H1 peptides. The blot was stripped and reprobed for each successive analysis. Phosphorylated H1 and unphosphorylated H1 isoforms are labeled. B. Phosphatase treatment abolishes the signal recognized by anti-phosphorylated H1 antiserum. C. Unpurified anti-unphosphorylated H1 antiserum recognizes both phosphorylated and unphosphorylated H1s. D. Anti-unphosphorylated H1 antibodies purified by immunoadsorption demonstrate high specificity for unphosphorylated H1.