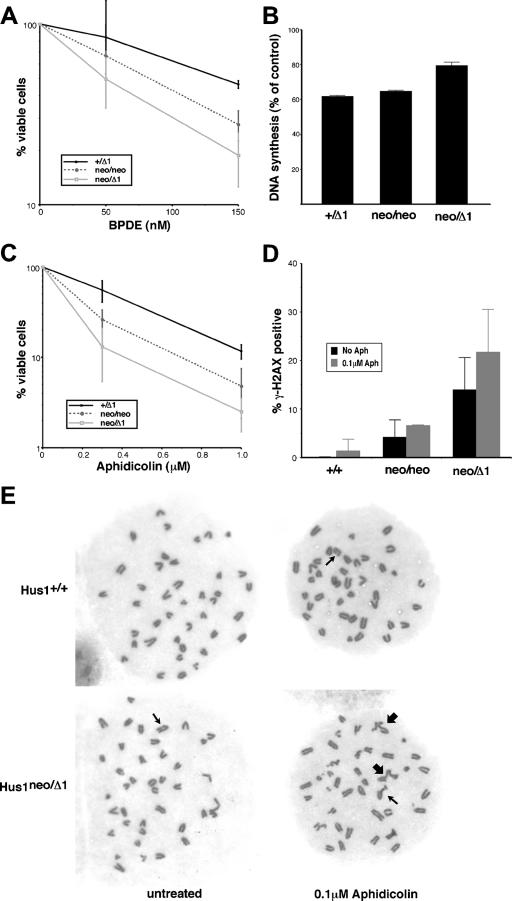
FIG. 5.
Defective responses to DNA damage and replication blockage in cells expressing reduced levels of Hus1. (A) Cell viability following BPDE treatment. Hus1+/Δ1, Hus1neo/neo, and Hus1neo/Δ1 MEFs at passage one were treated with BPDE, and cell viability was measured 72 h posttreatment. The percentage of viable cells, relative to mock-treated control cultures, is plotted. Values are the mean of triplicate samples, with error bars representing the standard deviation. (B) DNA synthesis following BPDE treatment. Hus1+/Δ1, Hus1neo/neo, and Hus1neo/Δ1 MEFs at passage two were treated with BPDE and then assayed for DNA synthesis 1 h later by measurement of radiolabeled thymidine incorporation. The percentage of DNA synthesis, relative to that for matched mock-treated control cells, is shown. Values are the mean of triplicate samples, with error bars representing the standard deviation. (C) Cell viability following aphidicolin treatment. Hus1+/Δ1, Hus1neo/neo, and Hus1neo/Δ1 MEFs at passage one were treated with aphidicolin for 24 h, and cell viability was measured 72 h posttreatment. The percentage of viable cells, relative to mock-treated control cultures, is plotted. Values are the mean of triplicate samples, with error bars representing the standard deviation. (D) γ-H2AX accumulation following aphidicolin treatment. Hus1+/+, Hus1neo/neo, and Hus1neo/Δ1 cells at passage three were treated with 0.1 μM aphidicolin for 24 h, and the percentage of γ-H2AX-positive cells was then determined by indirect immunofluorescence. Values are the mean for three independent 40× microscope fields, with error bars representing the standard deviation. (E) Representative metaphase spreads from aphidicolin-treated MEFs. Hus1+/+ and Hus1neo/Δ1 MEFs at passage two were mock treated or treated with 0.1 μM aphidicolin for 24 h. Metaphase spreads were then prepared, stained with Giemsa, and imaged with a 100× objective lens. Thin arrows indicate chromosome gaps and breaks, while thick arrows indicate chromatid interchanges.