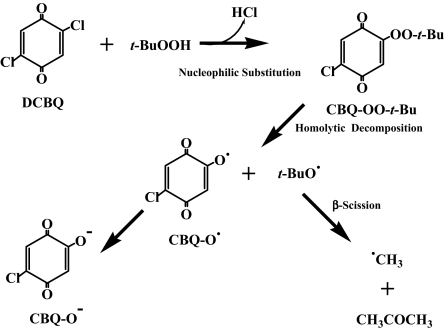
Scheme 1.
Proposed mechanism for DCBQ-mediated t-BuOOH decomposition and formation of t-BuO• and •CH3: A nucleophilic reaction may take place between DCBQ and t-BuOOH, forming a chloro-t-butylperoxyl-1,4-benzoquinone (CBQ-OO-t-Bu) intermediate, which can decompose homolytically to produce t-BuO• and 2-chloro-5-hydroxy-1,4-benzoquinone radical (CBQ-O•). CBQ-O• then disproportionate to form the ionic form of 2-chloro-5-hydroxy-1,4-benzoquinone (CBQ-O−), and •CH3 can be produced through β-scission of t-BuO•.