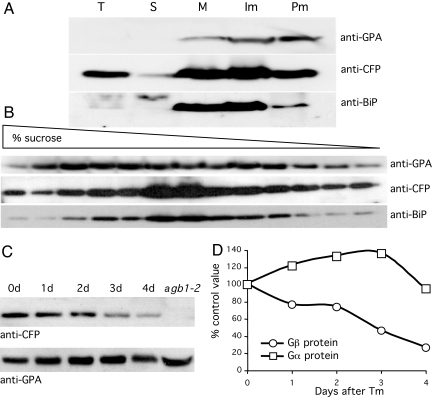
Fig. 4.
Gβ protein colocalizes with BiP in the ER and is degraded during the UPR. (A) Aliquots of total cell extracts (T) and soluble (S) supernatant and pelleted microsomal (M) fractions as well the upper plasma membrane (Pm) fraction and lower intracellular membrane (Im) fractions after aqueous two-phase partitioning were analyzed together by PAGE and Western blotting using anti-GPA antibodies to detect Gα protein, anti-CFP antibodies to detect the CFP-Gβ fusion protein in extracts of transgenic plants expressing a Gβ-CFP fusion protein from the native Gβ promoter, and anti-BiP antibodies for the ER markers BiP1 and 2. (B) The Im fraction was sedimented through a 10–50% sucrose density gradient, and aliquots of each fraction were analyzed by PAGE and Western blotting as in A. (C) Gβ and Gα proteins were detected as in A in total extracts from Gβ-CFP transgenic plants injected with Tm at 30 μg/ml and sampled after 1–4 days. A comparable protein extract from agb1-2 mutant plants was used in the last lane and shows that the Gα protein is expressed in the absence of the Gβ protein. (D) Band intensities from C are expressed as a percentage of control values.