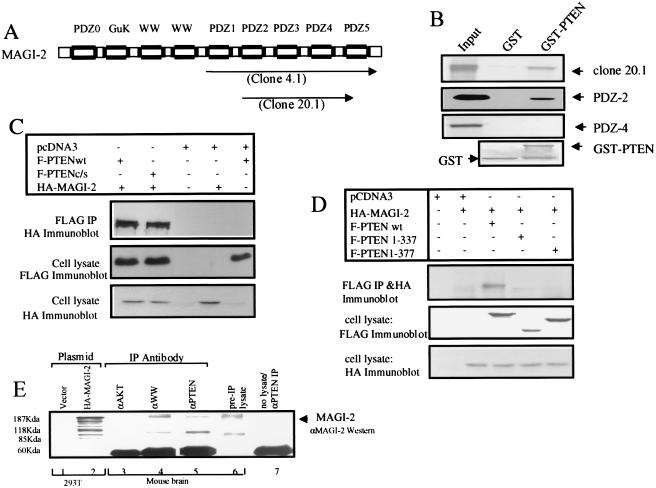
Figure 1.
PTEN binds MAGI-2 through a PDZ domain-mediated interaction. (A) Structure of MAGI-2. The two clones isolated in the two-hybrid screen (clones 4.1 and 20.1) are indicated by arrows. PDZ0 indicates a probable PDZ domain that does not have the consensus GLGF sequence. The numbering of PDZ domains 1–5 is based on the previously published nomenclature for MAGI-1 (30). GuK, guanylate kinase domain. (B) 35S-labeled in vitro transcribed/translated MAGI-2 protein from clone 20.1 and clones of individual PDZ domains 2 or 4 was pulled down with GST alone or full-length PTEN-GST beads. Input represents 20% of the protein used. The bottom panel shows Coomassie staining of protein bound to beads. (C) 293T cells were transfected with wild-type (wt) or mutant FLAG-PTEN constructs and HA-MAGI-2. Lysates were immunoprecipitated (IP) with anti-FLAG antibody and immunoblotted with anti-HA antibody 12CA5 to detect MAGI-2 or anti-FLAG antibody to detect PTEN. (D) Transfection and immunoprecipitation (IP) were performed in 293T cells as indicated in C. (E) MAGI-2 was immunoprecipitated (IP) from the homogenate of fresh mouse brain (lane 4) by using antisera raised against the WW domains of S-SCAM (synaptic scaffolding molecule), the rat homologue of MAGI-2 (27). PTEN was immunoprecipitated (lane 5) by using polyclonal antisera (28) or an independently derived PTEN antibody (19) (not shown). Anti-Akt polyclonal antisera (New England Biolabs) (lane 3), pre-IP lysate (lane 6), and no lysate (lane 7) were used as controls. Immunoblots were analyzed by using rabbit polyclonal antisera against GST-MAGI-2.