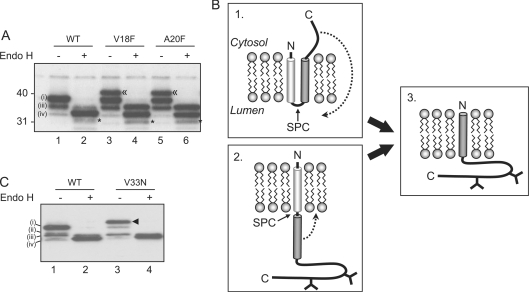
Figure 2. The SPC mediates cleavage of the torsinA signal sequence.
(A) Wild-type (WT) torsinA or cleavage site mutants (V18F and A20F) were transiently transfected into HeLa cells. Cell lysates were treated with endo H or mock treated and analysed by Western blotting with affinity purified anti-torsinA. A higher molecular mass species generated by the cleavage site mutants is labelled ≪. An additional product seen after endo H treatment is labelled *. A singly glycosylated intermediate (ii) was not apparent in this experiment. (B) Potential mechanisms for torsinA to adopt a type II orientation. (C) Wild-type torsinA (WT) or a form containing a third glycosylation site (V33N) was transiently transfected into HeLa cells, and lysates treated as in (A). An additional species representing triply glycosylated torsinA V33N is labelled ◀.