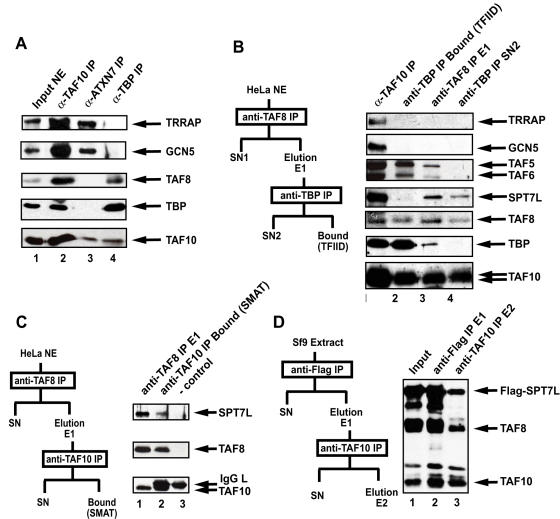
Figure 5. TAF8 is not a subunit of TFTC/STAGA type complexes, but is present in TFIID and a novel TAF8-, TAF10- and SPT7L-containing complex that can also be formed in vitro.
(A) The indicated transcription factors were immunoprecipitated using specific mAbs (1H8 anti-TAF10; 2A10 anti-ATXN7 and 2C1 anti-TBP) from HeLa cell nuclear extract (NE) and eluted by an excess of peptides against which the mAbs were raised. Eluted protein complexes were separated by SDS-PAGE and analysed by western blot with the indicated antibodies. (B) TAF8-containing protein complexes were purified from HeLa NE according to the scheme shown on the left of the panel. The eluate obtained after the first anti-TAF8 IP using the 2TAU 2B8 mAb (anti-TAF8 IP E1; lane 3) and the supernatant obtained after the TAF8-containing complexes were depleted in TFIID using the anti-TBP 2C1 mAb (SN2; lane 4). They were separated along with complexes obtained after an anti-TAF10 IP (TFIID and TFTC together; lane 1) or with a highly purified TFIID fraction (lane 2) on SDS-PAGE and analysed with the indicated antibodies by western blot. (C) TAF8 containing complexes were purified from HeLa NE according to the scheme on the left of the panel. TAF10-containing complexes were re-precipitated from the eluate obtained after the first anti-TAF8 IP (E1, lane 1) extensively washed and loaded after boiling the beads (lane 2) with loading buffer on a SDS-PAGE. The migration of the antibody alone (-control) is shown in lane 3. Proteins were analyzed with the indicated antibodies by western blot. (D) TAF8, TAF10 and Flag-SPT7L were co-expressed in Sf9 cells. WCEs were made and proteins were subjected to two successive immunoprecipitations and elutions by peptide competition according to the schemes shown on the left of the panel. Input and eluted protein complexes were separated by SDS-PAGE and analysed with the indicated antibodies by western blot.