Figure 4.
Interaction between TAC1 and the BT2 Promoter.
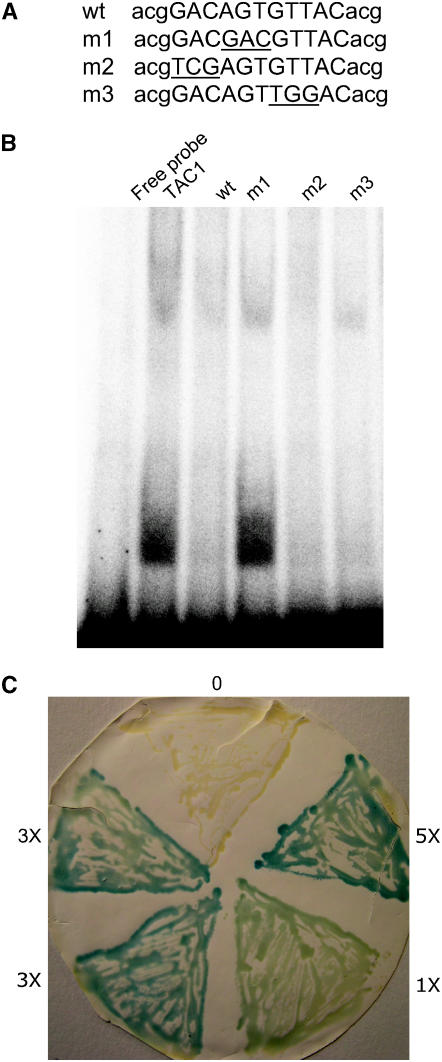
(A) Sequences of oligonucleotides used for mobility-shift assays. An 11-nucleotide region from the BT2 promoter that shared 10 nucleotides with a binding site for another plant zinc-finger protein (see text) is shown in uppercase letters. Three arbitrary nucleotides flanking this sequence are shown in lowercase letters. Wild-type sequence for one strand is shown on the top. Oligonucleotides m1, m2, and m3 carry mutations at the underlined positions.
(B) Mobility-shift assays with the TAC1 protein and BT2 oligonucleotides. Radiolabeled wild-type oligonucleotide alone is designated Free Probe. TAC1 binds to this oligonucleotide, resulting in a slower mobility (TAC1). Cold wild-type oligonucleotide at 50-fold excess effectively competes for TAC1 binding to the labeled probe (lane wt). Mutation of the central AGT triplet eliminates this competition (lane m1). Oligonucleotides with mutations at three nucleotides on either side of the AGT core compete as effectively as the wild-type sequence (lanes m2 and m3).
(C) Interaction between TAC1 and the BT2 promoter sequence in yeast one-hybrid assays. Zero, one, three, or five copies of the wild-type oligonucleotide shown in (A) were cloned upstream of the lacZ gene in the pYC7 plasmid and transformed into yeast expressing TAC1. lacZ activity, judged by staining with X-Gal, correlates with the number of putative binding sites.