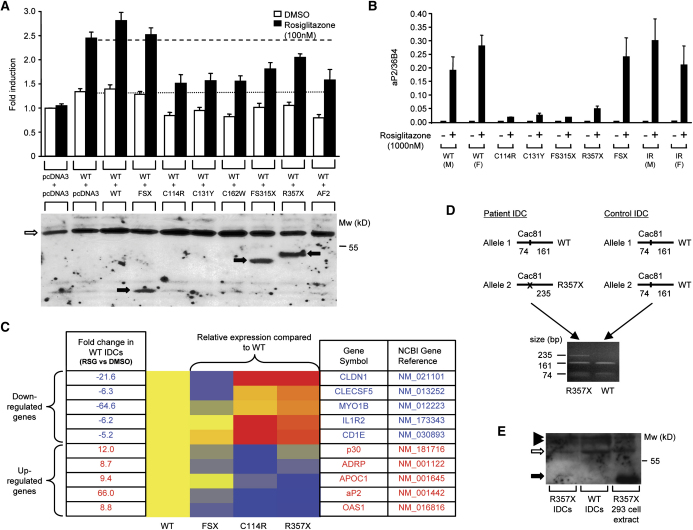
Figure 2.
PPARγ mutants exhibit dominant-negative activity
A) The C114R, C131Y, C162W, FS315X, and R357X PPARγ mutants inhibit transactivation by wild-type (WT) receptor, comparably to AF2, an artificial PPARγ mutant described previously, whereas the FSX mutant lacks dominant-negative activity (upper panel). 3T3-L1 cells were cotransfected with 33 ng of WT receptor plus an equal amount of either empty (pcDNA3) or WT or mutant expression vector, together with 265 ng of human aP2LUC reporter plasmid and 65 ng of the internal control plasmid Bos-β-gal. The dotted and dashed lines denote transcriptional activity of WT receptor in the absence and presence of ligand respectively. Results are expessed as fold induction relative to empty vector (pcDNA3 + pcDNA3) and represent the mean ± SEM of at least three independent experiments in triplicate. Expression of wild-type and mutant receptor proteins was confirmed by Western blotting (lower panel) and the positions of WT, C114R, C131Y, C162W, and AF2 PPARγ (open arrow) and FSX, FS315X and R357X truncation mutants (solid arrows) are indicated.
B and C) Ligand-dependent regulation of PPARγ target genes in IDCs from subjects with PPARγ mutations. (B) Induction of the aP2 gene by rosiglitazone, measured by qPCR, is markedly impaired in IDCs derived from subjects with the C114R, C131Y, FS315X, and R357X mutations, compared to responses in cells from normal (WT), severely insulin resistant (IR) subjects without mutations in PPARγ and cells with the FSX, haploinsufficient, mutation. Results represent the mean ± SEM of more than three independent experiments in triplicate, except for cells with the FS315X mutation where a single representative experiment is shown. (C) Relative expression of several PPARγ target genes (5 downregulated and 5 upregulated) in WT and mutation-containing (FSX, C114R, R357X) IDCs, measured by qPCR using TLDA. Red indicates higher, and blue lower, levels of gene expression relative to rosiglitazone-treated (1000 nM) WT cells, whose responses are uniformly designated yellow. Fold changes in expression of each gene in rosiglitazone (RSG) versus vehicle (DMSO) treated WT cells are also listed.
D and E) The R357X PPARγ mutant is expressed in IDCs. (D) PPARγ cDNA flanking the R357 codon was amplified by RT-PCR in IDCs from patient S5 and a control subject. Cac81 enzyme digestion of PCR products derived from the WT allele yields two fragments (161 and 74 bp), whereas abolition of this restriction site in the R357X mutant allele yields a larger 235 bp product. (E) Whole-cell lysates of WT and R357X mutant IDCs and 293EBNA cells transfected with R357X mutant were immunoprecipitated and Western blotted. The positions of WT PPARγ (open arrow), R357X (solid arrow), and nonspecific bands (solid arrowheads) are indicated.