Abstract
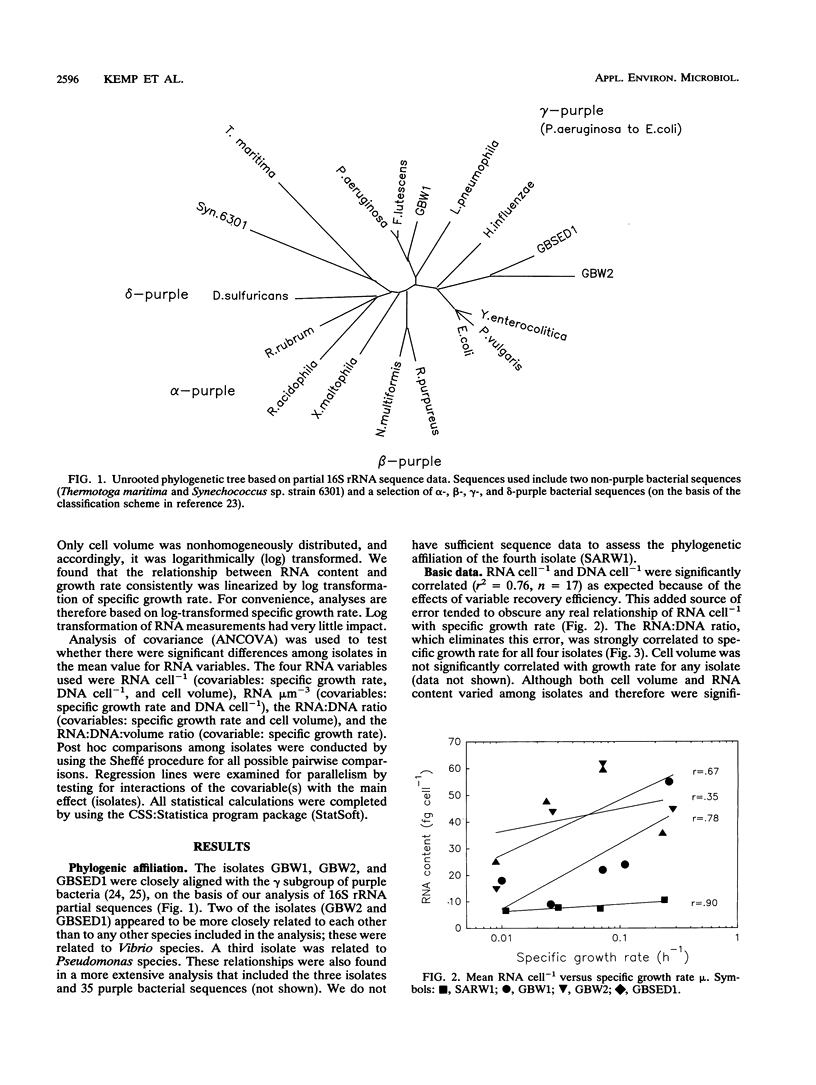
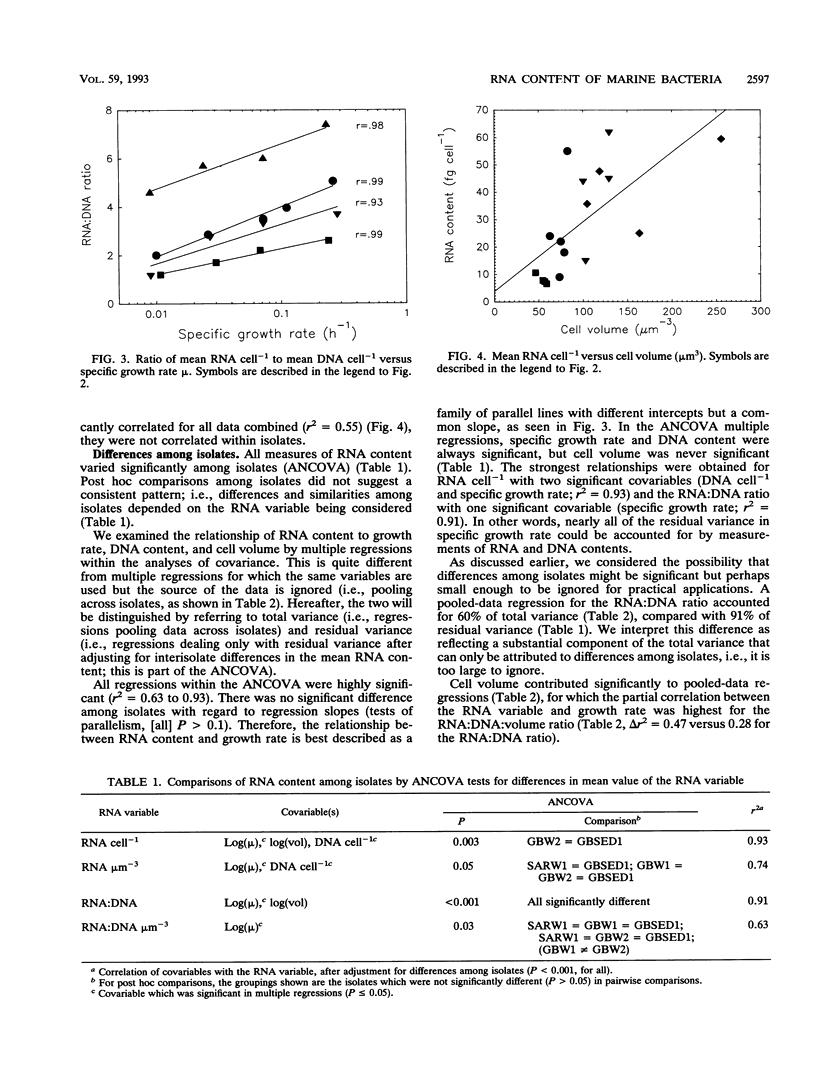
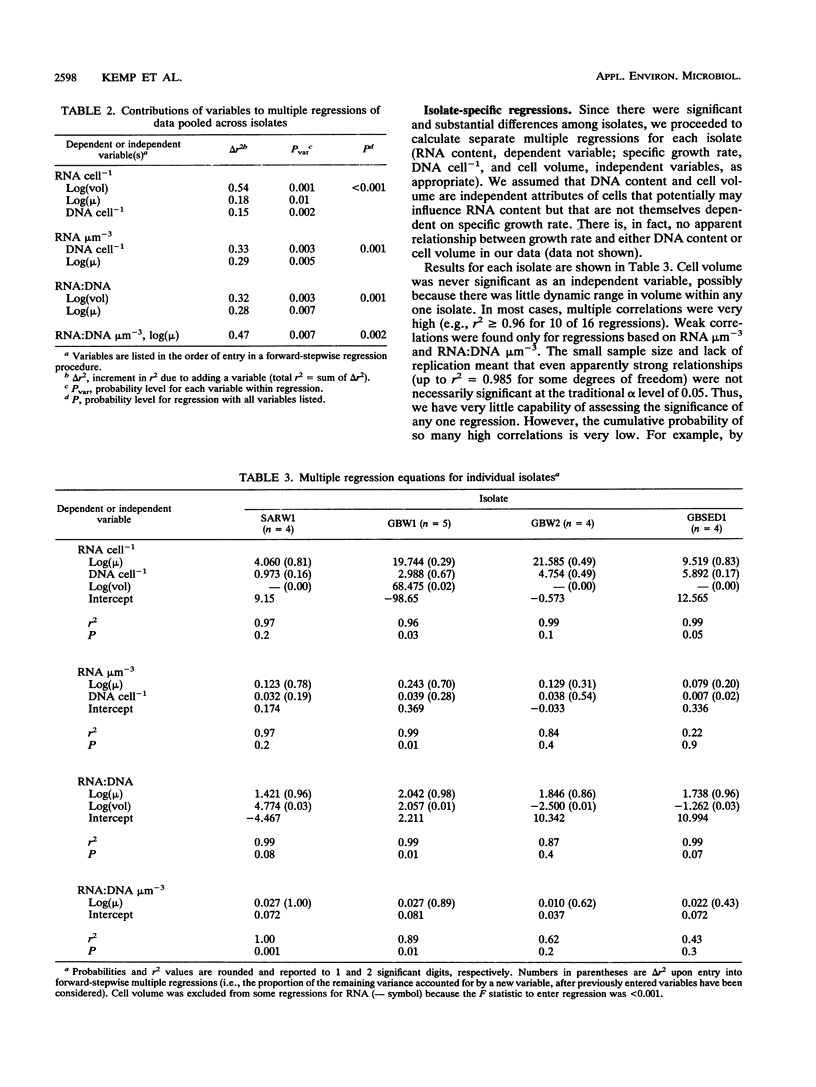
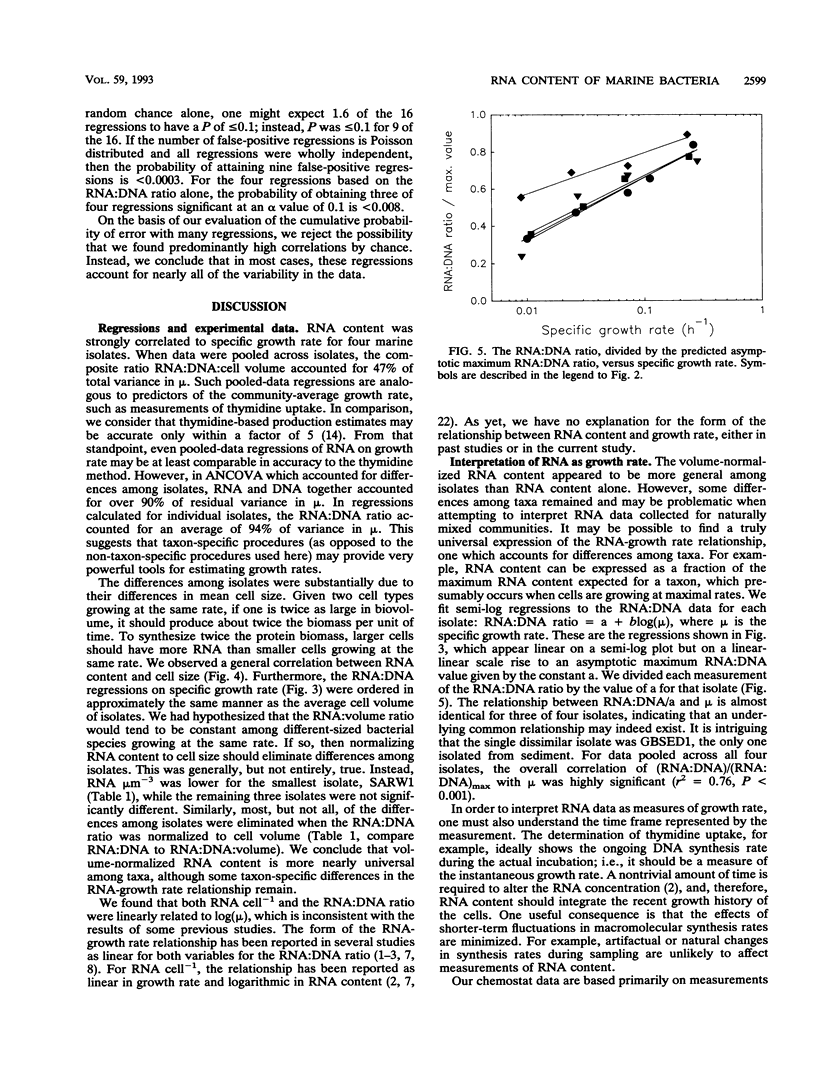
In past studies of enteric bacteria such as Escherichia coli, various measures of cellular RNA content have been shown to be strongly correlated with growth rate. We examined this correlation for four marine bacterial isolates. Isolates were grown in chemostats at four or five dilution rates, yielding growth rates that spanned the range typically determined for marine bacterial communities in nature (μ = 0.01 to 0.25 h-1). All measures of RNA content (RNA cell-1, RNA:biovolume ratio, RNA:DNA ratio, RNA:DNA:biovolume ratio) were significantly different among isolates. Normalizing RNA content to cell volume substantially reduced, but did not eliminate, these differences. On average, the correlation between μ and the RNA:DNA ratio accounted for 94% of variance when isolates were considered individually. For data pooled across isolates (analogous to an average measurement for a community), the ratio of RNA:DNA μm-3 (cell volume) accounted for nearly half of variance in μ (r2 = 0.47). The maximum RNA:DNA ratio for each isolate was extrapolated from regressions. The regression of (RNA:DNA)/(RNA:DNA)max on μ was highly significant (r2 = 0.76 for data pooled across four isolates) and virtually identical for three of the four isolates, perhaps reflecting an underlying common relationship between RNA content and growth rate. The dissimilar isolate was the only one derived from sediment. Cellular RNA content is likely to be a useful predictor of growth rate for slowly growing marine bacteria but in practice may be most successful when applied at the level of individual species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis B. D., Luger S. M., Tai P. C. Role of ribosome degradation in the death of starved Escherichia coli cells. J Bacteriol. 1986 May;166(2):439–445. doi: 10.1128/jb.166.2.439-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Pedersen S. Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of the macromolecular biosynthetic apparatus with substrates and catalytic components. Microbiol Rev. 1990 Jun;54(2):89–100. doi: 10.1128/mr.54.2.89-100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Lee S., Fuhrman J. A. DNA hybridization to compare species compositions of natural bacterioplankton assemblages. Appl Environ Microbiol. 1990 Mar;56(3):739–746. doi: 10.1128/aem.56.3.739-746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S., DuMouchelle E., Koniuch D., Oberleas D. A simple fluorometric method for the determination of RNA and DNA in tissues. J Lab Clin Med. 1972 Oct;80(4):598–602. [PubMed] [Google Scholar]
- Ryals J., Little R., Bremer H. Temperature dependence of RNA synthesis parameters in Escherichia coli. J Bacteriol. 1982 Aug;151(2):879–887. doi: 10.1128/jb.151.2.879-887.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Schmidt T. M., DeLong E. F., Pace N. R. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J Bacteriol. 1991 Jul;173(14):4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]


