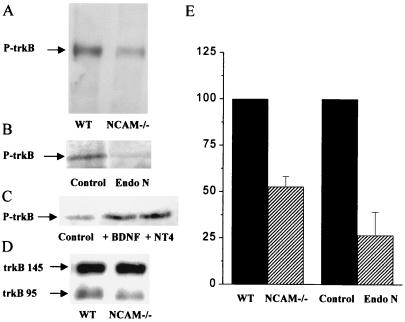
Figure 4.
Reduced trkB phosphorylation in NCAM knockout mice and organotypic slice cultures treated with Endo-N. (A) Immunoblot obtained by using a monoclonal anti-trkB receptor antibody after immunoprecipitation with a monoclonal phosphospecific antibody recognizing phosphorylated trkB in wild-type (WT) and NCAM knockout mice (NCAM −/−). (B) Immunoblot obtained in the same way after immunoprecipitation with the antiphosphotyrosine antibody from control and Endo-N-treated slice cultures. (C) Immunoblot obtained after immunoprecipitation from slices prepared from NCAM-deficient mice in control conditions (Left), and after treatment for 30 min with either 100 ng/ml BDNF (Middle) or 200 ng/ml NT4/5 (Right). (D) Western blot carried out with a monoclonal trkB antibody before immunoprecipitation and showing that the amount of trkB receptor protein was comparable in wild-type (WT) and NCAM knockout mice (NCAM −/−). (E) Quantitative assessment by densitometry analysis of autoradiographic films of trkB phosphorylation in wild-type and NCAM knockout mice and in control and Endo-N-treated slice cultures as revealed in three different experiments for each condition. Differences are statistically significant (P < 0.05; n = 3; t test).