Abstract
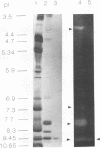
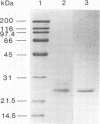
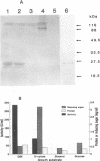
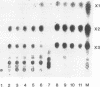
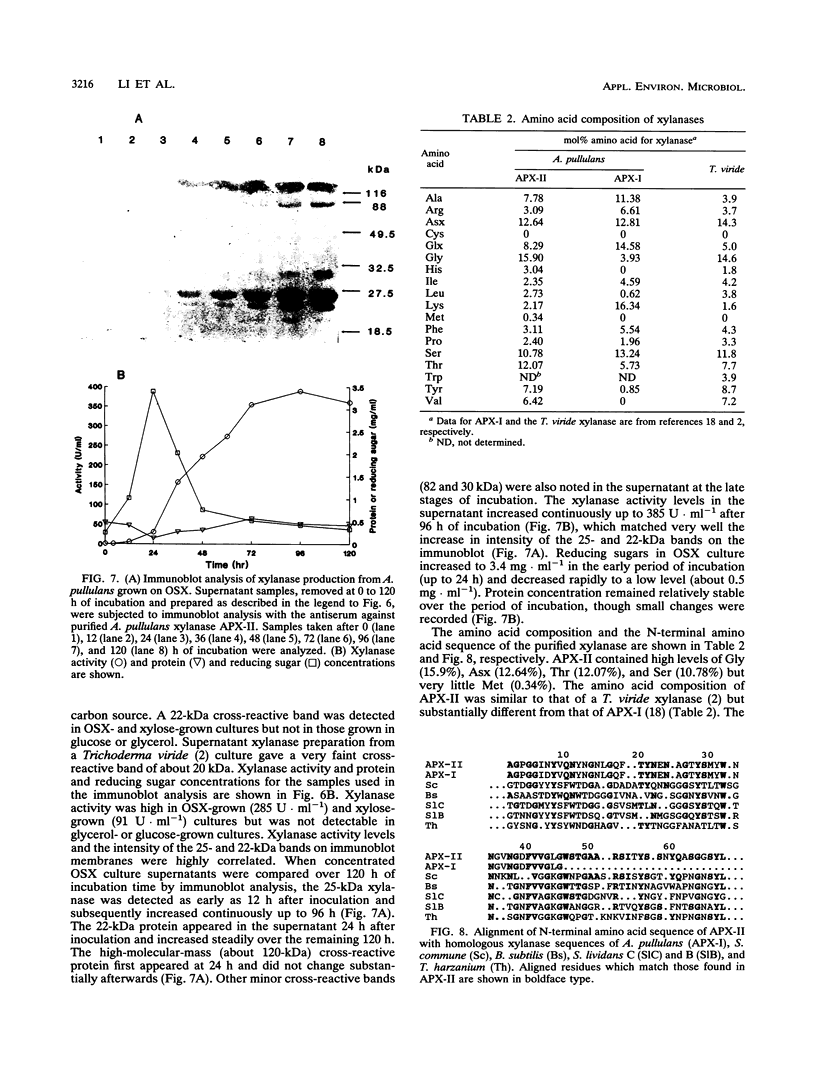
Aureobasidium pullulans Y-2311-1 produced four major xylanases (EC 3.2.1.8) with pI values of 4.0, 7.3, 7.9, and 9.4 as revealed by isoelectric focusing and zymogram analysis when grown for 4 days on 1.0% oat spelt xylan. The enzyme with a pI of 9.4 was purified by ammonium sulfate precipitation, chromatography on a DEAE-Sephadex A-50 column, and gel filtration with a Sephadex G-75 column. The enzyme had a mass of about 25 kDa as determined by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration chromatography. The purified enzyme had a Km of 7.6 mg . ml(-1) and Vmax of 2,650 micromol . min(-1) . mg(-1) for birchwood xylan at 28 degrees C and pH 4.5. It lacked activity towards carboxymethylcellulose, cellobiose, starch, mannan, p-nitrophenyl (pNP)-beta-D-xylopyranoside, pNP-beta-D-glucopyranoside, pNP-alpha-D-glucopyranoside, pNP-beta-D-cellobioside, pNP-beta-D-fucopyranoside, or pNP-alpha-D-galactopyranoside. The predominant end products of birchwood xylan or xylohexaose hydrolysis were xylobiose and xylose. The enzyme had the highest activity of pH 4.8 and 54 degrees C. Sixty percent of the activity remained after the enzyme had been incubated at 55 degrees C and pH 4.5 for 30 min. The sequence of the first 68 amino acid residues at the amino terminus showed homology to those of several other xylonases. Immunoblot analysis with antiserum raised against the purified xylanase revealed that two immunologically related polypeptides of 25 and 22 kDa were produced in A. pullulans cultures containing oat spelt xylan or xylose as carbon sources but not in cultures containing glycerol or glucose.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dean J. F., Anderson J. D. Ethylene Biosynthesis-Inducing Xylanase : II. Purification and Physical Characterization of the Enzyme Produced by Trichoderma viride. Plant Physiol. 1991 Jan;95(1):316–323. doi: 10.1104/pp.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski A. C., Jeffries T. W. Production, Purification, and Characterization of beta-(1-4)-Endoxylanase of Streptomyces roseiscleroticus. Appl Environ Microbiol. 1991 Apr;57(4):987–992. doi: 10.1128/aem.57.4.987-992.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluepfel D., Vats-Mehta S., Aumont F., Shareck F., Morosoli R. Purification and characterization of a new xylanase (xylanase B) produced by Streptomyces lividans 66. Biochem J. 1990 Apr 1;267(1):45–50. doi: 10.1042/bj2670045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T., Ohkoshi A., Horikoshi K. Molecular cloning and expression of a xylanase gene of alkalophilic Aeromonas sp. no. 212 in Escherichia coli. J Gen Microbiol. 1985 Oct;131(10):2825–2830. doi: 10.1099/00221287-131-10-2825. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leathers T. D. Color Variants of Aureobasidium pullulans Overproduce Xylanase with Extremely High Specific Activity. Appl Environ Microbiol. 1986 Nov;52(5):1026–1030. doi: 10.1128/aem.52.5.1026-1030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer J. C., Nakas J. P. Simple, sensitive zymogram technique for detection of xylanase activity in polyacrylamide gels. Appl Environ Microbiol. 1990 Jun;56(6):1516–1517. doi: 10.1128/aem.56.6.1516-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareck F., Roy C., Yaguchi M., Morosoli R., Kluepfel D. Sequences of three genes specifying xylanases in Streptomyces lividans. Gene. 1991 Oct 30;107(1):75–82. doi: 10.1016/0378-1119(91)90299-q. [DOI] [PubMed] [Google Scholar]
- Tsujibo H., Miyamoto K., Kuda T., Minami K., Sakamoto T., Hasegawa T., Inamori Y. Purification, properties, and partial amino acid sequences of thermostable xylanases from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol. 1992 Jan;58(1):371–375. doi: 10.1128/aem.58.1.371-375.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K., Tan L. U., Saddler J. N. Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev. 1988 Sep;52(3):305–317. doi: 10.1128/mr.52.3.305-317.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones W. A., Woods D. R. Nucleotide sequence of a Clostridium acetobutylicum P262 xylanase gene (xynB). Nucleic Acids Res. 1990 Apr 25;18(8):2179–2179. doi: 10.1093/nar/18.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]