Abstract
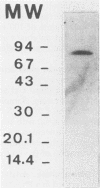
The extracellular alpha-amylase (1,4-alpha-D-glucanglucanohydrolase; EC 3.2.1.1) from Clostridium acetobutylicum ATCC 824 was purified to homogeneity by anion-exchange chromatography (mono Q) and gel filtration (Superose 12). The enzyme had an isoelectric point of 4.7 and a molecular weight of 84,000, as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It was a monomeric protein, the 19-amino-acid N terminus of which displayed 42% homology with the Bacillus subtilis saccharifying alpha-amylase. The amino acid composition of the enzyme showed a high number of acidic and hydrophobic residues and only one cysteine residue per mole. The activity of the alpha-amylase was not stimulated by calcium ions (or other metal ions) or inhibited by EDTA, although the enzyme contained seven calcium atoms per molecule. alpha-Amylase activity on soluble starch was optimal at pH 5.6 and 45 degrees C. The alpha-amylase was stable at an acidic pH but very sensitive to thermal inactivation. It hydrolyzed soluble starch, with a Km of 3.6 g . liter-1 and a Kcat of 122 mol of reducing sugars . s-1 . mol-1. The alpha-amylase showed greater activity with high-molecular-weight substrates than with low-molecular-weight maltooligosaccharides, hydrolyzed glycogen and pullulan slowly, but did not hydrolyze dextran or cyclodextrins. The major end products of maltohexaose degradation were glucose, maltose, and maltotriose; maltotetraose and maltopentaose were formed as intermediate products. Twenty seven percent of the glucoamylase activity generally detected in the culture supernatant of C. acetobutylicum can be attributed to the alpha-amylase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcock E. R., Woods D. R. Carboxymethyl cellulase and cellobiase production by Clostridium acetobutylicum in an industrial fermentation medium. Appl Environ Microbiol. 1981 Feb;41(2):539–541. doi: 10.1128/aem.41.2.539-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antranikian G., Herzberg C., Gottschalk G. Production of Thermostable alpha-Amylase, Pullulanase, and alpha-Glucosidase in Continuous Culture by a New Clostridium Isolate. Appl Environ Microbiol. 1987 Jul;53(7):1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R. R. A specific method for quantitative determination of glucose. Anal Biochem. 1966 Feb;14(2):258–260. doi: 10.1016/0003-2697(66)90134-5. [DOI] [PubMed] [Google Scholar]
- Buonocore V., Caporale C., De Rosa M., Gambacorta A. Stable, inducible thermoacidophilic alpha-amylase from Bacillus acidocaldarius. J Bacteriol. 1976 Nov;128(2):515–521. doi: 10.1128/jb.128.2.515-521.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and Characterization of Extracellular Amylolytic Enzymes from the Yeast Filobasidium capsuligenum. Appl Environ Microbiol. 1985 Dec;50(6):1474–1482. doi: 10.1128/aem.50.6.1474-1482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and characterization of extracellular alpha-amylase and glucoamylase from the yeast Candida antarctica CBS 6678. Eur J Biochem. 1987 May 4;164(3):643–654. doi: 10.1111/j.1432-1033.1987.tb11175.x. [DOI] [PubMed] [Google Scholar]
- ENGLARD S., SINGER T. P. Physicochemical studies on beta-amylase. J Biol Chem. 1950 Nov;187(1):213–219. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- FRENCH D., KNAPP D. W. The maltase of Clostridium acetobutylicum; its specificity range and mode of action. J Biol Chem. 1950 Dec;187(2):463–471. [PubMed] [Google Scholar]
- HSIU J., FISCHER E. H., STEIN E. A. ALPHA-AMYLASES AS CALCIUM-METALLOENZYMES. II. CALCIUM AND THE CATALYTIC ACTIVITY. Biochemistry. 1964 Jan;3:61–66. doi: 10.1021/bi00889a011. [DOI] [PubMed] [Google Scholar]
- Hockenhull D. J., Herbert D. The amylase and maltase of Clostridium acetobutylicum. Biochem J. 1945;39(1):102–106. doi: 10.1042/bj0390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H., Fietzek P. P., Lampen J. O. N-terminal amino acid sequence of Bacillus licheniformis alpha-amylase: comparison with Bacillus amyloliquefaciens and Bacillus subtilis Enzymes. J Bacteriol. 1982 Jan;149(1):372–373. doi: 10.1128/jb.149.1.372-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Cellulolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Aug;50(2):220–228. doi: 10.1128/aem.50.2.220-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Xylanolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Oct;50(4):1068–1076. doi: 10.1128/aem.50.4.1068-1076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Rattray J. B. Purification and Characterization of Two Endoxylanases from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1987 Apr;53(4):644–650. doi: 10.1128/aem.53.4.644-650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. H., Keay L. Purification and characterization of the amylase of B. subtilis NRRL B3411. Biotechnol Bioeng. 1970 Mar;12(2):251–271. doi: 10.1002/bit.260120207. [DOI] [PubMed] [Google Scholar]
- SCOTT D., HEDRICK L. R. The amylase of Clostridium acetobutylicum. J Bacteriol. 1952 Jun;63(6):795–803. doi: 10.1128/jb.63.6.795-803.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D., HEDRICK L. The amylase of Clostridium acetobutylicum. II. Adsorption. Appl Microbiol. 1959 May;7(3):135–138. doi: 10.1128/am.7.3.135-138.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Toda H., Kato I., Narita K. Correlation of the masked sulfhydryl group with the essential calcium in taka-amylase A. J Biochem. 1968 Mar;63(3):295–301. [PubMed] [Google Scholar]
- Toda H., Narita K. Correlation of the sulfhydryl group with the essential calcium in Bacillus subtilis saccharifying alpha-amylase. J Biochem. 1968 Mar;63(3):302–307. [PubMed] [Google Scholar]
- VALLEE B. L., STEIN E. A., SUMERWELL W. N., FISCHER E. H. Metal content of alpha-amylases of various origins. J Biol Chem. 1959 Nov;234:2901–2905. [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuuki T., Nomura T., Tezuka H., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a gene coding for heat- and pH-stable alpha-amylase of Bacillus licheniformis: comparison of the amino acid sequences of three bacterial liquefying alpha-amylases deduced from the DNA sequences. J Biochem. 1985 Nov;98(5):1147–1156. doi: 10.1093/oxfordjournals.jbchem.a135381. [DOI] [PubMed] [Google Scholar]