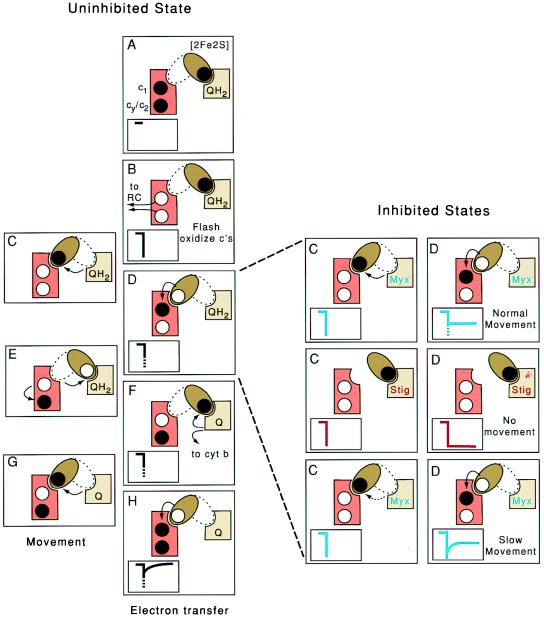
Figure 2.
Proposed intracomplex electron shuttle mechanism in cyt bc1. (Left) Schematic representations of the different steps of electron transfer (A, B, D, F, and H) and movement (C, E, and G) in the uninhibited cyt bc1. The [2Fe2S] cluster domain, the cyt c complement (c1 + cy/c2) and the Qo site are shown as a brown ellipsoid, orange rectangle, and tan square, respectively. Black dots represent electrons located on the [2Fe2S] cluster and the cyt c heme complement before flash oxidation (A). Arrows refer to electron transfers between cofactors and movement of the [2Fe2S] cluster domain between its Qo and c1 positions. (Insets) Kinetics of cyt c oxidation (downward deflection) and cyt c reduction (upward deflection). The dotted parts of the traces in D, F, and H indicate the predicted unresolved fast phase. On flash activation of the reaction center and oxidation of the cyt c complement (B), the [2Fe2S] cluster domain is proposed to move from its Qo to c1 position (C), transfer an electron to cyt c1 of the cyt c complement (D), and return to the Qo position (E). Then, QH2 is oxidized to ubiquinone (Q); one electron is transferred to the [2Fe2S] cluster; and the other is transferred to the low potential cyt b chain (F). The [2Fe2S] cluster domain moves back to the c1 position (G) where it transfers another electron (H). (Right) Representation of the steps C (movement) and D (electron transfer) shown in Left in inhibited states. In the presence of the Qo site inhibitor myxothiazol (Top), normal movement of the [2Fe2S] cluster domain will yield partial reduction of the cyt c complement with an unresolved fast phase (blue traces). In the presence of stigmatellin or in a mutant locking the [2Fe2S] cluster domain in Qo position (Middle), the cyt c complement should remain completely oxidized (red traces), whereas in a mutant slowing the movement (Bottom), this fast phase should be time-resolved (blue traces).