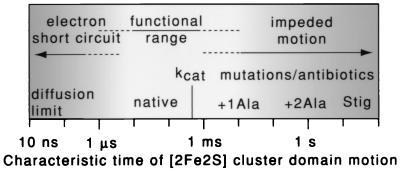
Figure 5.
Functional range of the [2Fe2S] cluster domain movement. The time scale of the [2Fe2S] cluster domain movement seems poised between two extremes of failure. Slowing the movement, as in the +1Ala mutant, to just less than the normal rate of Qo site catalysis kcat (characteristic turnover time of 600 μs) constrains the cyt bc1 turnover and compromises growth. Impeding the motion more drastically with stigmatellin, or as in the +2Ala mutant, blocks cyt bc1 catalysis and photosynthetic growth. Conversely, at faster times, simulations suggest that speeding the motion toward the physical diffusion limits (10 ns) may enable wasteful electron transfer and short circuit the redox-energy conversion mechanism.