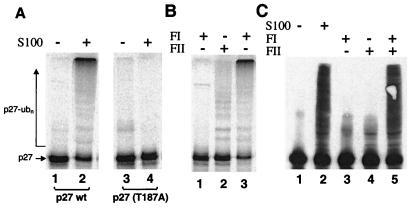
Figure 1.
In vitro ubiquitination of p27Kip1. (A) HeLa S100 contains a ubiquitin ligase activity specific for p27 phosphorylated on Thr-187. Reaction mixtures contained S100 extract as indicated and [35S]His6-p27 wild type (lanes 1 and 2) or mutant (T187A) (lanes 3 and 4). After incubation for 1 h at 30°C, [35S]His6-p27 and its polyubiquitinated forms were resolved on a 12% Tris-glycine SDS-gel, and analyzed by phosphorimaging. (B) Both FI and FII are required for robust ubiquination of in vitro-translated p27. [35S]His6-p27 was incubated with FI and/or FII as indicated, and reactions were analyzed as in A. Note that FII alone supported a basal level of ubiquitination (lane 2), which was likely caused by reticulocyte lysate in the reaction. (C) Ubiquitination of bacterially expressed p27 is FI and FII dependent. Reactions contained S100 extract, FI, and/or FII as indicated. GST-p27 and its polyubiquitinated forms were resolved on an 8% Tricine SDS-gel and immunoblotted by using anti-p27 antibodies. Note that robust p27 conjugate formation was detected only in reactions containing S100 or both FI and FII (lanes 2 and 5).