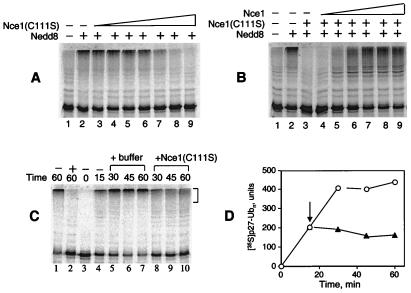
Figure 4.
The Nce1 C111S mutant exerts a dominant negative effect on p27 ubiquitination. (A) Ubiquitination of in vitro-translated [35S]His6-p27 was carried out with FII, 0.29 μM Nedd8 (lanes 2–9), and Nce1 mutant C111S in increasing concentrations: 0.08, 0.2, 0.3, 0.5, 1, 2, and 4 μM (lanes 3–9). (B) Wild-type Nce1 reverses inhibition by Nce1 C111S. Ubiquitination of in vitro-translated [35S]His6-p27 was carried out by using FII, 0.29 μM Nedd8 (lanes 2–9), 4 μM Nce1 C111S (lanes 3–9), and increasing wild-type Nce1: 0.02, 0.1, 0.2, 0.5, 1, and 2 μM (lanes 4–9, respectively). (C) Continuous Nce1 activity is required to sustain the p27 ubiquitination reaction. Ubiquitination of in vitro-translated [35S]His6-p27 was carried out by using S100 extract. Two reactions were incubated in the absence (lane 1) or presence (lane 2) of 4 μM Nce1 C111S for 60 min. The reaction in lane 3 was immediately mixed with Ni-NTA beads and kept on ice until completion of the experiment. The remaining reactions were incubated at 30°C for 15 min and either terminated by the addition of Ni-NTA and chilling on ice (lane 4), or mixed with Nce1 C111S (final concentration 4 μM, lanes 8–10), or mock buffer (lanes 5–7). Reactions were further incubated 30°C for an additional 15, 30, or 45 min before termination. (D) Polyubiquitinated [35S]p27 reaction products (shown with brackets in C) were quantified by phosphorimage analysis. The arrow indicates the addition of Nce1 C111S (▴) or mock buffer (○).